
(12��) �ظ�����(Na2Cr2O7)�㷺���ںϳ����ϡ�ýȾ���ȡ��Ը�����(��Ҫ�ɷ�ΪCr2O3������FeO��Al2O3��SiO2������)Ϊԭ����ȡ�ظ����Ƶ�����ͼ���£�
��ش��������⡣
(1)��I��ֻ��һ���Լ�������Һ��pH��Ӧѡ��___________(����)��
��A��ϡ���ᡡ B�������ƹ��塡 C������������Һ
(2)I�У�������ҺpH�������Һ��pH��С�����ܵ���W���������ܽ⣬ԭ����
��_______________________________________________________(�����ӷ���ʽ��ʾ)��
(3)���У�Na2CrO4ת��ΪNa2Cr2O7�����ӷ�Ӧ���£���2CrO42-(��ɫ)+2H��Cr2O72-(�Ⱥ�ɫ)+H2O
�ٸ÷�Ӧ________������ԭ��Ӧ(��ǡ����ǡ�)����Ӧ��ƽ�ⳣ������ʽ K=__________��
������Na2Cr2O7��Һ(�Ⱥ�ɫ)�м�������NaOH���壬��Һ_______ (����)��
����A�����ɫ�� B����ɫ���䡡 C���Ⱥ�ɫ����
����֪��25��ʱ��Ag2CrO4��Ksp=1.12��10-12��Ag2Cr2O7��Ksp=2��10-7��25��ʱ����Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ��_____________________��
(1)A��(2)Al(OH)3+3H+��Al3++3H2O��
![]() (3)�ٲ��ǡ�
(3)�ٲ��ǡ�
��
��A���� ��Ag2CrO4
����:��1��I��Ҫ������Һ��pHֵ����Ҫ������Һ��pHֵ������Ӧѡ�������Լ�����ѡA��
��2�������Һ��pH��С����H�������ˣ�Al(OH)3���ܽ⣬���ӷ���ʽΪAl(OH)3+3H+��Al3++3H2O
��3���٢������ӷ���ʽ��Ԫ�ػ��ϼ۵ı仯�����Բ���������ԭ��Ӧ��
![]() ƽ�ⳣ��K����ʽΪ��
ƽ�ⳣ��K����ʽΪ��
�ڼ��� ����NaOH���壬ƽ�������ƶ�����ɫ�гȺ�ɫ��ɻ�ɫ��ѡA
�������֪������Ag2Cr2O7��������Ksp֪Ag2CrO4�����ܣ�Na2Cr2O7��Һ�Լ��ԣ��ڼ���������Ag2Cr2O7����ת���ɸ����ܵ�Ag2CrO4��


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������2010��������Ĵ��ʼ쿼�ԣ���ѧ������ ���ͣ������
(12��) �ظ�����(Na2Cr2O7)�㷺���ںϳ����ϡ�ýȾ���ȡ��Ը�����(��Ҫ�ɷ�ΪCr2O3������FeO��Al2O3��SiO2������)Ϊԭ����ȡ�ظ����Ƶ�����ͼ���£�
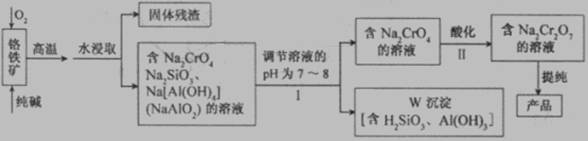 ��ش��������⡣
��ش��������⡣
(1)��I��ֻ��һ���Լ�������Һ��pH��Ӧѡ��___________(����)��
��A��ϡ���ᡡ B�������ƹ��塡 C������������Һ
(2)I�У�������ҺpH�������Һ��pH��С�����ܵ���W���������ܽ⣬ԭ����
��_______________________________________________________ (�����ӷ���ʽ��ʾ)��
(3)���У�Na2CrO4ת��ΪNa2Cr2O7�����ӷ�Ӧ���£���2CrO42-
(��ɫ)+2H��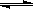 Cr2O72-
(�Ⱥ�ɫ)+H2O
Cr2O72-
(�Ⱥ�ɫ)+H2O
�ٸ÷�Ӧ________������ԭ��Ӧ(��ǡ����ǡ�)����Ӧ��ƽ�ⳣ������ʽ K=__________��
������Na2Cr2O7��Һ(�Ⱥ�ɫ)�м�������NaOH���壬��Һ_______ (����)��
����A�����ɫ�� B����ɫ���䡡 C���Ⱥ�ɫ����
����֪��25��ʱ��Ag2CrO4��Ksp=1.12��10-12��Ag2Cr2O7��Ksp=2��10-7��25��ʱ����Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ��_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com