
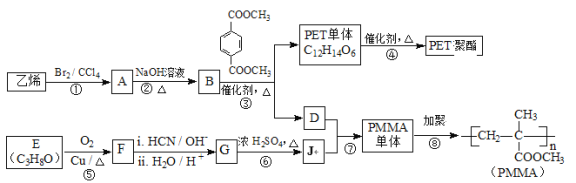
����Ŀ���߷��Ӳ���PET������֬��PMMA�ĺϳ�·������ͼ��ʾ��
��֪����. RCOOR��+R����OH ![]() RCOOR����+R��OH
RCOOR����+R��OH
��. ![]()
��ش��������⣺
(1) A�ķ���ʽ��___________��
(2) E�Ľṹ��ʽ��________________����Ӧ�ķ�Ӧ������____________ ��
(3)�����ʵ�����G�ֱ�������Na��NaHCO3��Һ��Ӧ�����ɵ���������ͬ״���������Ϊ______��
(4) J�й����ŵ�����Ϊ__________��
(5)д����Ӧ�ݵĻ�ѧ����ʽ��__________________��
(6)д��һ�ַ�������������PMAA�����ͬ���칹��Ľṹ��ʽ��_________________��
a.����PMAA��������й�����b.��������������Һ��Ӧ��������c.����3�ֲ�ͬ��ѧ��������ԭ��
���𰸡�C2H4Br2 CH3CH(OH)CH3 ��ȥ��Ӧ 1:1 ̼̼˫�����Ȼ� 2CH3CH(OH)CH3 +O2![]() 2CH3COCH3+2H2O HCOOCH=C(CH3)2
2CH3COCH3+2H2O HCOOCH=C(CH3)2
��������
��PMMA�Ľṹ����֪PMMA����ΪCH2=C(CH3)COOCH3����D��J�ֱ�ΪCH2=C(CH3)COOH��CH3OH�е�һ�֣���ϩ���巢���ӳɷ�Ӧ����AΪCH2BrCH2Br��A��NaOHˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH��������ϢI��PET�������ʽ����֪PET����Ϊ ����DΪCH3OH��JΪCH2=C(CH3)COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ
����DΪCH3OH��JΪCH2=C(CH3)COOH��PET���巢����ϢI�н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ ��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ(CH3)2COHCOOH����FΪCH3COCH3��EΪCH3CH(OH)CH3���ݴ˽��
��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����GΪ(CH3)2COHCOOH����FΪCH3COCH3��EΪCH3CH(OH)CH3���ݴ˽��
(1)������A�Ľṹ��ʽΪCH2BrCH2Br��֪A�ķ���ʽ��C2H4Br2���ʴ�Ϊ��C2H4Br2��
(2)���ɷ�����֪E�Ľṹ��ʽ��CH3CH(OH)CH3����Ӧ�ķ�Ӧ��������ȥ��Ӧ���ʴ�Ϊ��CH3CH(OH)CH3����ȥ��
(3)��G�Ľṹ��ʽΪ(CH3)2COHCOOH�������ǻ����Ȼ����Ȼ�������Na��NaHCO3��Һ�����ǻ�ֻ����Na��Ӧ�����Ե����ʵ�����G�ֱ�������Na��NaHCO3��Һ��Ӧ�����ɵ���������ͬ״���������Ϊ��1:1���ʴ�Ϊ��1:1��
(4)���ɷ�����֪J�ĽṹΪCH2=C(CH3)COOH������J�й����ŵ�����Ϊ��̼̼˫�����Ȼ����ʴ�Ϊ��̼̼˫�����Ȼ���
(5)����Ӧ����E[CH3CH(OH)CH3]���������ȵ������·������Ĵ���������F(CH3COCH3)���ʷ�Ӧ�ݵĻ�ѧ����ʽ��2CH3CH(OH)CH3 +O2![]() 2CH3COCH3+2H2O��
2CH3COCH3+2H2O��
(6)��������������a.����PMAA��������й����ţ�������̼̼˫����������b.��������������Һ��Ӧ����������˵�����м����γɵ�������c.����3�ֲ�ͬ��ѧ��������ԭ�ӣ������ĵ�PMAA�����ͬ���칹��Ľṹ��ʽΪ��HCOOCH=C(CH3)2���ʴ�ΪHCOOCH=C(CH3)2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������[(NH4)2Fe(SO4)2]�Ƿ�����ѧ�е���Ҫ�Լ����ڲ�ͬ�¶��¼��ȷֽ���ﲻͬ�������ͼʵ��װ�ã��г�װ����ȥ������500��ʱ������������A�е�������������ֽ���ȫ��ȷ���ֽ����ijɷ֡�

(1)Bװ�õ�������________________________________��
(2)ʵ���У��۲쵽C������������D���а�ɫ�������ɣ���ȷ�������ж���______���������д��D�з�����Ӧ�����ӷ���ʽ____________________����ȥ��C���Ƿ��ܵó�ͬ�����۲�������ԭ��__________________________��
(3)A�й���ֽ�����NH3��д����������;________________________________________��
(4)A�й�����ȫ�ֽ���Ϊ����ɫ��ĩ��ijͬѧ���ʵ����֤����������ΪFe2O3������FeO������ɱ����ݡ�(�Լ���������ѡ)
ʵ�鲽�� | Ԥ������ | ���� |
��ȡ����A�в��������Թ��У���������ϡ���ᣬ�����ʹ����ȫ�ܽ�; ��______________________________________________________ | ����������ΪFe2O3 |
(5) ��ʵ��ķ�����֤C��Һ����NH4+___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ��ʱ�����c��d�������ֱ�μӷ�̪��Һ��c��������Һ��죬����˵����ȷ����( )
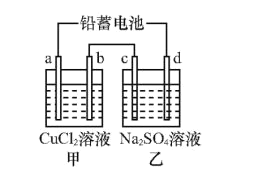
A.Ǧ���ظ�����ӦΪ![]()
B.c����������b��������
C.��a��Ϊͭ����׳�b��������ͭ
D.�ҳ���c��d��Ϊ���Ե缫,����������ҺpH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ά����P�Ľṹ��ͼ��ʾ������RΪ�����������й���ά����P��������ȷ����
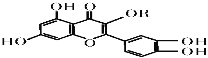
A.�����еĹ��������ǻ���̼̼˫�����Ѽ�������
B.��RΪ����������ʵķ���ʽ���Ա�ʾΪC16H12O7
C.1 mol�û�������������NaOH Ϊ5mol
D.1 mol�û��������������ˮ�е���5 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���밴Ҫ����գ�
��1��д��![]() �к��������ŵ�����_______________��
�к��������ŵ�����_______________��
��2��![]() ����__________����
����__________����
��3��CH2=CH��CH3�ڴ����������������ɾۺ���ķ�Ӧ����ʽΪ________��
��4��д��ʵ��������Ȳ�Ļ�ѧ��Ӧ����ʽ_________________��
��5���ٳ�ȡ3.4gij�л�������A����ȫȼ�պ�����1.8g H2O��8.8g CO2����֪���л��������������������ܶ�Ϊ68������л���ķ���ʽΪ_________________��
�ڸ��л���A�ĺ˴Ź������ͺ���������£�

���Ʋ��л���A�Ľṹ��ʽΪ_________________________��
����A����ͬ���л����ͬ���칹�����A����_________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾʾ��ͼ������˵������ȷ����

A. ��Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪC(s)+H2O(g)![]() CO(g)+H2(g) ��H=(b-a) kJ��mol-1
CO(g)+H2(g) ��H=(b-a) kJ��mol-1
B. �÷�Ӧ���̷�Ӧ��ϼ����յ���������������ɼ��ų�������
C. a mol C��a mol H2O��Ӧ����a mol CO��a mol H2���յ�����һ��Ϊ131.3a kJ
D. 1 mol C(g)��2 mol H��1 mol Oת���1 mol CO(g)��1 mol H2(g)�ų�������Ϊa kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. 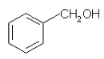 ��
��![]() ������ͬ�Ĺ����ţ���Ϊͬϵ��
������ͬ�Ĺ����ţ���Ϊͬϵ��
B. 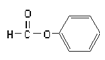 ����ȩ�࣬������Ϊ��CHO
����ȩ�࣬������Ϊ��CHO
C. ![]() ������Ϊ��2���һ���1����ϩ
������Ϊ��2���һ���1����ϩ
D. ![]() ������Ϊ��2������1��3������ϩ
������Ϊ��2������1��3������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PVAc��һ�־��������Ե���֬���ɺϳ���Ҫ�߷��Ӳ���M���ϳ�·�����£�
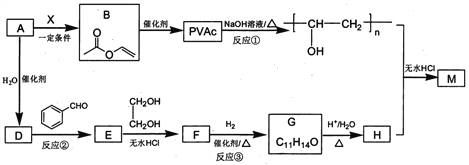
��֪��R��R�@��R�@�@ΪHԭ�ӻ�����
I. R'CHO+ R"CH2CHO![]()
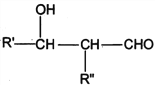
![]()
![]()
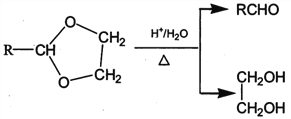
II. RCHO+![]()
![]()
��1����״���£�4.48L��̬��A��������5.2g����A�Ľṹ��ʽΪ___________________��
��2����֪A��BΪ�ӳɷ�Ӧ����X�Ľṹ��ʽΪ_______��B�й����ŵ�������_________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ______________________��
��4��E��ʹ������Ȼ�̼��Һ��ɫ����Ӧ�ڵķ�Ӧ�Լ���������_______________________��
��5����Ӧ�۵Ļ�ѧ����ʽΪ____________________________��
��6����E��F��G��H��ת�������У��Ҷ�����������__________________________��
��7����֪M�������г������⣬��������Ԫ��״�ṹ����M�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ����20mL 0.1 mol/L��CH3COOH��Һ����εμ�0.1 mol/L��NaOH��Һ������NaOH��Һ���������ҺpH�ı仯��ͼ��ʾ������˵����ȷ����
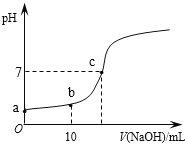
A. a���pH=1
B. b��ʱ��c (CH3COO��)=0.05mol/L
C. c��ʱ��V(NaOH)=20mL
D. ��Ӧ������![]() ��ֵ��������
��ֵ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com