��������1��ͬһ���ڴ����ң�Ԫ�صĵ�һ�����������VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�ͬһ������ϵ��£�Ԫ�صĵ�һ������С��
��2��N
60����������Nԭ�Ӻ��Ҽ����Ӷ���Ϊ3�ԣ�����Nԭ�ӵĵ��Ӷ���=3
+=4��
�ṹ���Ƶķ��ӣ��������γɹ��ۼ���ԭ�Ӱ뾶ԽС������Խ����Խ�ȶ���
��3���ṹ���ƣ�Ħ������Խ��������۷е�Խ�ߣ������Ӽ�����������������Ӽ���������ʹ�۷е����ߣ�
��4��C��C�γ�ԭ�Ӹ�����Ϊ1��3�ij�������ΪCO
32-������Cԭ�Ӽ۲���Ӷ�����µ��Ӷԣ�ȷ����ռ乹�ͣ�
��5����ͬ�ܲ���������������ܲ�����������ϴ�ʧȥ��ͬ�ܲ����ʱ�����ܻᷢ��ͻԾ��
��6����ԭ�Ӻ����ж��ٸ����Ӿ;��ж������˶�״̬��ij�ֽ���Ԫ�غ��������29���˶�״̬�����Ԫ��Ϊ29��Ԫ��Cu��
���
�⣺��1��ͬһ������ϵ��£�Ԫ�صĵ�һ������С�����Ե�һ�����ܴ�С��N��P��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ������������������ʵ�һ�����ܴ�С��S��Si�������ڵ�VA��Ԫ��np
3�����ڰ����״̬����һ�����ܴ�������Ԫ�أ��ʵ�һ�����ܴ�С��P��S�����ϵ�һ�����ܴ�С��N��S��Si��
�ʴ�Ϊ��N��S��Si��
��2��N
60����������Nԭ�Ӻ��Ҽ����Ӷ���Ϊ3�ԣ�����Nԭ�ӵĵ��Ӷ���=3
+=4������Nԭ�ӵ��ӻ���ʽ��SP
3��
�ṹ���Ƶķ��ӣ��������γɹ��ۼ���ԭ�Ӱ뾶ԽС������Խ����Խ�ȶ���Cԭ�Ӱ뾶С��Siԭ�Ӱ뾶��C-C�����ܴ���Si-Si�����ܣ�����C
60 ���ȶ��Դ��� Si
60��
�ʴ�Ϊ��SP
3�����ڣ�
��3���ṹ���ƣ�Ħ������Խ��������۷е�Խ�ߣ����۷е�CH
4��SiH
4�������Ӽ�����������������Ӽ���������ʹ�۷е����ߣ���NH
3�۷е���ߣ�
�ʴ�Ϊ��CH
4��SiH
4��NH
3��NH
3���Ӽ�������۷е���ߣ�CH
4��SiH
4 ���Ӽ�ֻ�з��»�������Է�������CH
4��SiH
4 ���»���CH
4��SiH
4��
��4��C��O�γ�ԭ�Ӹ�����Ϊ1��3�ij�������ΪCO
32-��������Cԭ�ӵ��Ӷ���=
3+=3�����������Ŀռ乹��Ϊƽ�������Σ�
��CO
32-��Ϊ�ȵ�����ķ�����SO
3��BCl
3 ��BF
3��
�ʴ�Ϊ��ƽ�������Σ�SO
3��BCl
3 ��BF
3��
��5����ͬ�ܲ���������������ܲ�����������ϴ�ʧȥ��ͬ�ܲ����ʱ�����ܻᷢ��ͻԾ��NԪ�ع��������ܲ㣬�ڶ��ܲ���5�����ӣ�����NԪ�صĵ�����ͻ�� Ӧ�����ڵ�ʧ����������ʱ����������������ͻ�䣻
�ʴ�Ϊ������
��6��ԭ�Ӻ����ж��ٸ����Ӿ;��ж������˶�״̬��ij�ֽ���Ԫ�غ��������29���˶�״̬�����Ԫ��Ϊ29��Ԫ��Cu�����̬ԭ�Ӻ�������Ų�ʽΪ��1s
22s
22p
63s
23p
63d
104s
1�����Կ���ͭԭ�ӵĻ�̬ԭ�ӹ����s��p��d��������3����״��ͬ��ԭ�ӹ��������һ�����ӵ���Χ�����Ų�ʽΪ3d
10������Cu������֪����ԭ�ӵ���λ��=3��
8��=12��
ÿ�������к��е�ԭ����=8��
+6��
=4����ÿ������������m=64g/mol��
=
��ÿ�����������V=��a��4��10
-10��
3cm
3����Cu�ܶ�d=
=
����N
A=
mol
-1��
�ʴ�Ϊ��3�� 3d
10��12��
mol
-1��

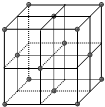 ̼�ж���ͬ�������壬�������н��ʯ��ʯī����C60������ϩ�����ӣ����ݱ���������ԱӦ�ü����ģ����ṹ����C60������N60���Ӻ�Si60���ӣ�
̼�ж���ͬ�������壬�������н��ʯ��ʯī����C60������ϩ�����ӣ����ݱ���������ԱӦ�ü����ģ����ṹ����C60������N60���Ӻ�Si60���ӣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
