
��14�֣��ζ����ǻ�ѧ�о��г��õĶ���ʵ�鷽����
��ij��ѧ��ȤС������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һ�ⶨ��Ũ�ȡ�
��ʵ����Ӧѡ�õ�ָʾ��Ϊ ��
�����в������²ⶨ���ƫ�ߵ��� ��
a����ʽ�ζ���������ˮ��ϴ��δ��������ϴ
b����ƿ������ˮ��ϴ��δ�ô�������������Һ��ϴ
c����ʽ�ζ��ܵζ�ǰ���촦�����ݣ��ζ���������ʧ
��Ī�ַ���һ�ֳ����ζ���.�ⶨij��Һ�ĵ�c(Cl��),��K2CrO4Ϊָʾ�����ñ���������Һ�ζ�����Һ[Ksp(AgCl)=1.56��10��10, Ksp(Ag2CrO4)=1.10��10��12,Ag2CrO4Ϊש��ɫ]
�ٵζ��յ�������� ��
�ڸõζ����˵�pH��Χ��6.5��10.5������Һ������δ��ڣ�c(NH4+)��0.05mol/Lʱ��Ӧ����Һ��pH������6.5��7.2���������й�˵������Ϊ��ȷ���� ��
a������ҺpH��6.5����ƽ��Cr2O72��+H2O 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�
b������Һ������δ��ڣ���pH��7.2ʱ�������������[Ag(NH3)2]+�������յ��ͺ�
c���ζ�ʱӦ����ҡ������ʹ��AgCl����������Cl����ʱ�ͷų�������ֹ�ζ��յ��ͺ�
��������ԭ�ζ���ˮ�������ij��÷��������ڲⶨ��ˮ�еĻ�ѧ����������λmg/L����ÿ��ˮ���л�ԭ�����ʱ�������O2����������ij��ȤС��ÿ��ȡ100mL��ˮ���������ữ����0.01667mol/LK2CrO7��Һ25.00mL��ʹˮ���еĻ�ԭ��������ȫ������Ȼ����0.1000mol/LFeSO4����Һ�ζ�ʣ���Cr2O72����ʵ�����ݼ�¼���£�
|
ʵ����� |
FeSO4��Һ���������/mL |
|
|
�ζ�ǰ |
��� |
|
|
1 |
0.10 |
16.20 |
|
2 |
0.30 |
15.31 |
|
3 |
0.20 |
15.19 |
�Իش��������⣺
��___Cr2O72��+____Fe2++____ ________==_____Cr3++_____Fe3++____H2O
�ڼ���÷�ˮ�Ļ�ѧ����������д��������̣��������һλС������
�Ţٷ�̪�����ȣ���2�֣���a��c��2�֣� �Ƣ�����ש��ɫ������2�֣���a��b��2�֣�
�Ǣ� Cr2O72��+ 6 Fe2++ 14 H+ == 2 Cr3++ 6 Fe3++ 7 H2O��2�֣� ��80.0mg/L��4�֣�
����������1����ǿ���ǿ��֮��ĵζ�����̪����Ⱦ�����ָʾ����
��a�൱��ϡ������ᣬ��������������ƫ�ⶨ���ƫ�ߣ�b����ȷ�IJ�������Ӱ�죻c�൱��������������ƫ�ⶨ���ƫ�ߣ���ѡac��
��2���ٵ�������ǡ�÷�Ӧ֮���ټ�����������������ɸ�����ש��ɫ�������ݴ˿��ж��յ㡣
�ڵζ�ʱ����ҡ��������ʹ��Һ�Ž����������ʵ��������c�Ǵ���ġ��������ȷ�ġ���ѡab��
��3���ٸ���������ԭ��Ӧ�е��ӵĵ�ʧ�غ������ƽ����Ԫ�صĻ��ϼ��ɣ�6�۽��͵���3�ۣ�����1mol�������õ�6mol���ӡ���1mol��ԭ����������ֻ��ʧȥ1mol���ӣ������������ͻ�ԭ�������ʵ���֮����1�U6����ʽΪCr2O72��+ 6 Fe2++ 14 H+ == 2 Cr3++ 6 Fe3++ 7 H2O��
������ʵ�����ĵĵ�����������Һ����ֱ���16.10ml��15.01ml��14.99ml����˵����һ��ʵ����ʧ�ܵġ�ȡ�����ε����ƽ��ֵΪ15.00ml��������K2CrO7�����ʵ�����2.5��10��4mol����˺ͻ�ԭ�����ʷ�Ӧ��K2CrO7�����ʵ�����4.1675��10��4mol��2.5��10��4mol��1.6675��10��4mol��ת�Ƶ�����1.6675��10��4mol��6��1.0��10��3mo��������Ҫ������1.0��10��3mo��4��2.5��10��4mol�����Է�ˮ�еĻ�ѧ��������2.5��10��4mol��32g/mol��1000mg/g��0.1L��80.0mg/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.ʵ�����Ʊ���ϩʱ���뽫�¶ȼ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
B.ȡ����������Һ��ϡ����ˮԡ���ȼ����Ӻ�����Ƶ�������ͭ��Һ���ȣ��۲������ж�����ˮ��IJ������Ƿ���������
C.�ڱ��м�����ˮ��������ã��۲������жϱ������Ƿ����̼̼˫��
D.ʵ�����Ʊ���������ʱ���Ƚ��Ҵ��������ϣ��ٰѻ��Һ���뵽Ũ������
E.��ȥ��������Һ��������NaCl���ɽ�װ�л��Һ�İ�Ĥ��������ˮ������
F.�к͵ζ�ʱ����ʽ�ζ���������ˮ��ϴ���κ������������еζ�
G.ʵ�����ýྻ���Թ���������Ӧʱ���ܽ��Թ�ֱ�ӷ��ھƾ��ƻ����ϼ���
��.��������̼���о��ɹ������˿Ƽ��Ľ��������õ绡���ϳɵ�̼���ܳ����д��������ʡ���̼������������̼���������������������ᴿ���䷴ӦʽΪ��
![]()
![]()
��1���˷�Ӧ����������______________������������______________��
��2��H2SO4��������Ӧ�б��ֳ�����������______________����ѡ���ţ���
A.���� B.������ C.��ˮ�� D.��ˮ��
��3����ƽ�Ļ�ѧ������Ϊ_________________��
��4��������Ӧ��������0.1 mol CO2���壬��ת�Ƶ��ӵ����ʵ�����____________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
��2����ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������()������ˮ��ClO2��
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:
������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ����Һ�е�
��ԭΪ
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ���ĺ������꣬�������м���������
��
��ԭΪ
����÷�Ӧ����������Ϊ (�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��16�֣���������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
(1)ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ

 ��
��
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ����_ __��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������_____________��

��װ��A�����ܽ�����Ķ����������壬�������ʢ��_______(����ĸ)��
a��20mL 60�����ˮ b��100mL��ˮ
c��100mL����ʳ��ˮ d��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ______________________
(2)��ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������( )������ˮ��ClO2��
)������ˮ��ClO2�� �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������____________________________��
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________��
������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________��
�۲���5��Ŀ����ʹ ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�ƺ����߸߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��
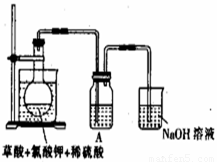
��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
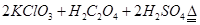
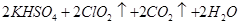
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
��2����ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������(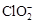 )������ˮ��ClO2��
)������ˮ��ClO2��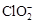 �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ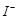 ����Һ�е�
����Һ�е�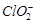 ��ԭΪ
��ԭΪ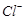 �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ��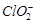 �ĺ������꣬�������м���������
�ĺ������꣬�������м���������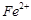 ��
��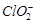 ��ԭΪ
��ԭΪ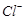 ����÷�Ӧ����������Ϊ
(�ѧʽ)
����÷�Ӧ����������Ϊ
(�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10������һ�и߶���ѧ�ڵڶ��ζο���ѧ�� ���ͣ�ʵ����
(14��)��������(ClO2)��Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��

(1)ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ


���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� (����ĸ)��
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12.25g KClO3��9g����(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ
(2)��ClO2������������ˮ(pHΪ5.5��6.5)������һ���������岻���������������(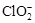 )������ˮ��ClO2��
)������ˮ��ClO2��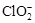 �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7.0��8.0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2.0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20.00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:

 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
��������ˮ�� �ĺ������꣬�������м���������
�ĺ������꣬�������м��������� ��
�� ��ԭΪ
��ԭΪ ����÷�Ӧ����������Ϊ
(�ѧʽ)
����÷�Ӧ����������Ϊ
(�ѧʽ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com