
��1����0.2mol��A����������ȫȼ��ʱ����CO2��H2O��1.2mol�������������2��2-�������飬��A�ĽṹʽΪ__________________��
��2��ijȲ����H2��ּӳ�����2��5-�������飬��Ȳ���Ľṹ��ʽΪ______________��
��3��ij��1mol��2mol HCl��ȫ�ӳɣ����ɵ��ȴ�������������4mol������Ӧ��������Ľṹ��ʽΪ________________��
��4��ij����A�������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й���6��������A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ_____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
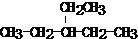
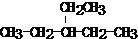
| �����Լ� | ���� | |
| C2H6 ��C2H2�� | ||
| C6H6��C6H5OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



 ���������ŵ�������
���������ŵ�������
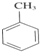 +3HNO3
+3HNO3
| ||
| ���� |
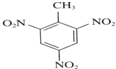 +3H2O��
+3H2O�� +3HNO3
+3HNO3
| ||
| ���� |
 +3H2O��
+3H2O��| ���� |
| ���� |
| �� |
| ���� |
| �� |
| ���� |
| ���� |
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)д�����и��л���Ľṹ��ʽ��
��1����0.2 mol��A����������ȫȼ��ʱ����CO2��H2O��1.2 mol�������������2��2-�������飬��A�ĽṹʽΪ___________��
��2������ʽΪC6H12��ijϩ�������е�̼ԭ�Ӷ���ͬһƽ���ϣ����ϩ���Ľṹ��ʽΪ______________________��
��3��ij����A�����ܶ�����ͬ״���������ܶȵ�64�������ⶨ֪A�����й���6������
����A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ______________________��
����A��Ȳ���������ӳɵIJ��A�Ľṹ��ʽΪ______________________��
��4��ij�����л������������Է�������Ϊ88.0����C����������Ϊ68.2%����H����������Ϊ13.6%����������ײⶨ����һ���ǻ����˴Ź���������ʾ�÷�������3�������������ֲ�ͬ��������ԭ�ӣ���д����ṹ��ʽ_____________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
(10��)д�����и��л���Ľṹ��ʽ��
��1����0.2 mol��A����������ȫȼ��ʱ����CO2��H2O��1.2 mol�������������2��2-�������飬��A�ĽṹʽΪ___________��
��2������ʽΪC6H12��ijϩ�������е�̼ԭ�Ӷ���ͬһƽ���ϣ����ϩ���Ľṹ��ʽΪ______________________��
��3��ij����A�����ܶ�����ͬ״���������ܶȵ�64�������ⶨ֪A�����й���6������
����A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ______________________��
����A��Ȳ���������ӳɵIJ��A�Ľṹ��ʽΪ______________________��
��4��ij�����л������������Է�������Ϊ88.0����C����������Ϊ68.2%����H����������Ϊ13.6%����������ײⶨ����һ���ǻ����˴Ź���������ʾ�÷�������3�������������ֲ�ͬ��������ԭ�ӣ���д����ṹ��ʽ_____________________.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com