
ij�о���ѧϰС������ữ����������Һ����I�����ڵĺ����Բ�������Ȥ��ͬѧ�Ǹ��ݱ�������˼��������·�����Ʋ�������ʵ��̽����
[�������]
����1�����ɵ�AgI������ˮ�����ܱ�HNO3������
����2��HNO3�������ԣ��ܽ�I��������I2��
[���ʵ�鷽������֤����]
��1����ͬѧ��KI��Һ�еμ����ữ��AgNO3��Һ�����л�ɫ�������ɡ���֤�˼���1��������д���йػ�ѧ����ʽ ��
��2����ͬѧ���ʵ����֤2�����������±������ݡ�
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| | ����Һ����������2������ ����Һ������������2�������� |
| ���� |
(12�֣�ÿ��2��)
��1��KI+AgNO3=AgI��+KNO3
��2��ȡ��ͬѧ���õ������ữ��AgNO3�μӵ�KI��������Һ��
��3����ͬ�⣬�����ữ��AgNO3���������ᣬ����֤�������Ƿ�������I-
��4������һ����FeCl3��Һ�еμӼ���KI��������Һ������Һ�Ƿ����
����������KI��KSCN��Һ�еμӼ���FeCl3��Һ������Һ�Ƿ��ȱ�����ɫ2Fe3++2I-=I2+2Fe2+
���������������2�����������ǿ�����Ժ͵�ʹ���۱���ɫ�����ԣ���4���������֤ʵ�鷽��ʱҪע�����һ�����������ǵ�����Ϊ��֤�������Ƿ���I2���Ƿ���Fe3+��
���㣺����ʵ�鷽������ƺ����ۣ����鿼�����ʵ��ͽ���ʵ�����۵�������


 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(18��)����������ڹ�ҵ��������;�dz��㷺��
��1��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ���ʵ��������480mLһ��Ũ�ȵ�Na2S2O3��Һ�����Ƹ���Һ���貣���������ձ�����Ͳ���������⣬����__________________��
��2��Na2S2O3���������軯��Ľⶾ������ҵ�ϳ�����Ʊ�Na2S2O3����Ӧԭ��Ϊ��
2Na2S+Na2CO3+4SO2 3Na2S2O3+CO2��ij�о�С����ʵ����ģ��ù�ҵԭ���Ʊ�Na2S2O3������ʵ��װ������:
3Na2S2O3+CO2��ij�о�С����ʵ����ģ��ù�ҵԭ���Ʊ�Na2S2O3������ʵ��װ������:
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ���B���Լ���________________������SO2����Ч�ʵ͵�ʵ��������B����Һ________________________��
��ʵ�����ʱ�����װ��C�е���Һ�����������ʣ�����һ��ΪNaOH����ʵ������и�װ���ڷ�����Ӧ�Ļ�ѧ����ʽ��_________________________�����������ʵ����ʵ�����ȣ������Һ��������Ũ�ȵĴ�С˳��Ϊ__________________________________��
�ۼ��豾ʵ�����õ�Na2CO3������NaC1��NaOH�����ʵ�鷽�����м��顣������±���
��֪������ʱCaCO3������Һ��pH=10��2��
��ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaC12��Һ����̪��Һ������ˮ��pH�ƣ��ձ����Թܡ���ͷ�ιܡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�������������������������������ʡ�����ʵ���������£�
��ش��������⣺
��1������1���õ��IJ���������������____________��
��2������Һ3��ȡ�������������ӷ���ʽΪ ��
��3������д����֤��Һ1����NH4+��ʵ����̣�_______________________________________________��
��4��ʵ������Fe2O3��CO��Ӧ����ȡ����Fe��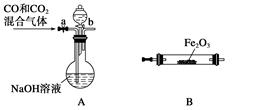

���밴���������ҵķ����������и�װ�ã�˳��ΪA�� ��
�ڼ��װ��A�����Եķ����� ��
���ڵ�ȼB���ľƾ���ǰ��Ӧ���еIJ�����_______________________________________����װ��C��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ���̲��ŷḻ����Դ����ˮ�ۺ����õ�����ͼ���¡�
��1����NaCl��ԭ�Ͽ��Եõ����ֲ�Ʒ��
�� ��ҵ����NaCl�Ʊ������ƵĻ�ѧ����ʽ��_______________________________��
�ڵ���Ȼ���ϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������Һ��ȫ���գ�����������Һ����һ�����ʣ�д����Ӧ�Ļ�ѧ����ʽ��____________________________��
��2����������κ��±ˮ���̺��ŷḻ��þ��Դ����������;���ɻ�ý���þ��
±ˮ Mg��OH��2
Mg��OH��2 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2 Mg
Mg
���У���MgCl2��6H2O��ȡ��ˮMgCl2�IJ���װ�ã�����̨���ƾ������ԣ����£�
����ͼ�У�װ��a�� �� ��˫�����͵�����ɡ�
��ѭ�����ʼ������� ��
����ȡ��ˮ�Ȼ�þ�������Ȼ�����ڵ������½��У�ԭ���� ��
��װ��b���������ʿ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijNa2CO3��Ʒ�л���һ������Na2SO4 (��������ᾧˮ����ij��ѧ��ȤС��������ַ����ⶨ����Ʒ��Na2CO3�������������Իش��������⡣
����һ���������з������Na2CO3���������Ĝy��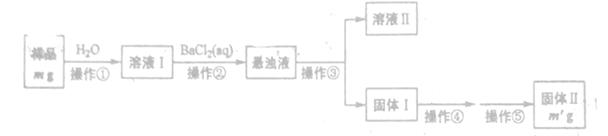
(1)�����ۺܵ͢����Ʒֱ�Ϊ_______��
(2)���������١����У�ʹ�õ�����������______(��������)��
(3)�жϲ����ڷ���ɵķ�����______
��������������ͼʵ��װ�ã��г�������ʡ�ԣ�.ѡ�������Լ�: a.Ũ����b.����NaHCO3��ҺC.6mol/L����D.2mol/L����, e.��ʯ��f. ��ˮCaCl2,�y����Ʒ��Na2CO3,������������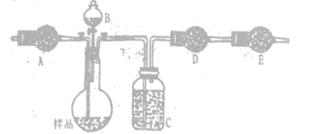
(4)��д���пո�
| ���� | �Լ� | ������Լ���Ŀ�� |
| A | | �������ʱϴȥCO2 |
| B | | ʹ��Ʒ��ַ�Ӧ�ų����� |
| C | a | |
| D | e | �������CO2 |
| E | e | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ����� ��
�ڲ���3����MgCO3��nH2O���������ӷ���ʽΪ ��
�۲���4�����Ƿ�ϴ�Ӹɾ��ķ����� ��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30�棬�����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
��ͼ������������� ��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ���� ��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ ���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��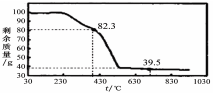
��������ºϳɵľ����У�n= ��ѡ�1��2��3��4��5����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ�r������ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������Ϊ���ijʳƷ���������κ�����ͨ����1kg��Ʒ�к�NaNO2��������)��ij�о�С���������������ʵ�鷽����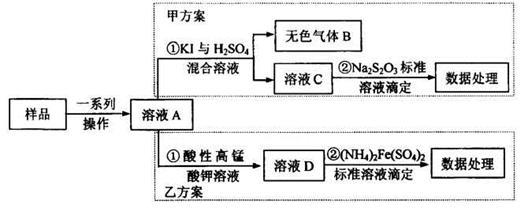
(1)��ɫ����B�������Ժ���ɫ��B�Ļ�ѧʽΪ_______д���������з�Ӧ�����ӷ���ʽ_______
(2)��ɲ���ƽ�ҷ������з�Ӧ�����ӷ���ʽ
MnO4-+ NO2-+ = Mn2++ NO3-+ ,
(3)�ҷ�������������100mL0.0010mol/L(NH4)2Fe(SO4)2����Һ������ȷ������Ʒ����������Ҫ�������У���Ͳ���ձ���_______������Һʱ�����ݵIJ���������______
(4)��ȡ��Ʒag�����ҷ������вⶨ��ȷ��ȡ12.00mL0.0005mol/L�����Ը��������Һ����ͯ������ҺA��Ӧ����Ӧ����Һ��0.0010mol/L(NH4)2Fe(SO4)2����Һ�ζ�����ɫ��Һ�պ���ȥ���ظ�����ʵ��2�Σ�ƽ������(NH4)2Fe(SO4)2��Һ10.00mL.��1kg��Ʒ��NaNO2������Ϊ_______mg.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ʊ�ˮ������������������� �����£�
�����£�
����һ����ˮ���ᾧ��Ͷ��������ƿ�У��ټ����ȱ��������ܽ������ˮ���Ȼ�����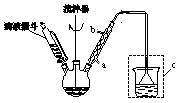
���������ͼ��ʾװ��װ���������ˮԡ���ȿ����¶���20��40��֮�䣬�ڽ����µμ�SOCl2����Ӧ�Ƶ�
ˮ�����ȡ��÷�ӦΪ��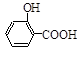 ��ˮ���ᣩ��SOCl2��
��ˮ���ᣩ��SOCl2�� ��ˮ�����ȣ���HCl����SO2��
��ˮ�����ȣ���HCl����SO2��
����������������ƿ�еĻ��Һ������80�棬�ټ������������[ ]���¶ȿ�����100�����ң����Ͻ��衣
]���¶ȿ�����100�����ң����Ͻ��衣
�����ģ����ˡ�����ѹ���ˣ��ƾ�ϴ�ӡ����
��1������һ�м������Ȼ����������� ��
��2��ʵ��ʱ���������е�ˮӦ�� �� ����ѡ�a����b������װ��c�������� ��
��3���������з�����Ӧ�Ļ�ѧ����ʽΪ���������������� ��������������������
��4�������ļ�ѹ���˲����У����ձ����������⣬������ʹ�õĹ����β��ϵ��������������������� ����������������
��5�������ļ�ѹ����ʱ����ʱ��ֽ�ᴩ�ף�������ֽ���Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ˮ��Դ�����ð�����ˮ�ĵ��������·����У����ܵ�����ˮ����
| A������ | B�����˷� | C���������� | D�����ӽ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com