
ЗлФЉзДЪдбљAЪЧгЩЕШЮяжЪЕФСПЕФMgOКЭFe2O3зщГЩЕФЛьКЯЮяЃЌ
НјааШчЯТЪЕбщЃКЃЈБОЬт13ЗжЃЉ
Ђй. ШЁЪЪСПAНјааТСШШЗДгІЃЌВњЮяжагаЕЅжЪBЩњГЩЃЛ
Ђк. СэШЁ20gAШЋВПШмгк0.15L6.0mol/LбЮЫсжаЃЌЕУШмвКCЃЛ
Ђл. НЋЂйжаЕУЕНЕФЕЅжЪBКЭШмвКCЗДгІЃЌЗХГі1.12LЃЈБъПіЃЉЦјЬхЁЃЭЌЪБЩњГЩШмвКDЃЌЛЙВаСєгаЙЬЬхЮяжЪBЃЛ
Ђм. гУKSCNШмвКМьВщЪБЃЌШмвКDВЛБфЩЋЁЃ
ЂХ. ЂйВњЮяжаЕФЕЅжЪЪЧ ЃпЃпЃпЃпЃпЃпЁЃИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ
ЂЦ. ЂкжаЫљЗЂЩњЕФИїЗДгІЕФЛЏбЇЗНГЬЪНЪЧ ЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃЛ
ЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЁЃ
ЂЧ. ЂлжаЫљЗЂЩњЕФИїЗДгІЕФРызгЗНГЬЪНЪЧ ЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃЛ
ЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЃпЁЃ
ЂШ. ШєШмвКDЕФЬхЛ§ШдЪгЮЊ0.15LЃЌдђИУШмвКжаc(Mg2+)ЮЊ ЃпЃпЃпЃпЃпЃпЃпЃпЃп
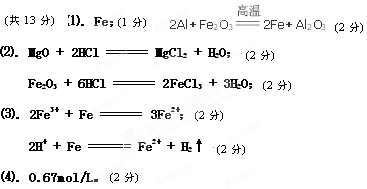
НтЮіЂХ. ЂйВњЮяжаЕФЕЅжЪЪЧFe,2AlЃЋFe2O3 2FeЃЋAl2O3
2FeЃЋAl2O3
ЂЦ. ЂкСэШЁ20gAжаКЌгаMgOКЭFe2O3,ЗЂЩњЕФЗДгІЪЧ;MgOЃЋ2HCl=MgCl2ЃЋH2O,Fe2O3ЃЋ6HCl=2FeCl3ЃЋ3H2O;
ЂЧНЋЂйжаЕУЕНЕФЕЅжЪBЮЊFe,КЭШмвКCжаFeCl3МАЙ§СПЕФHClЗДгІЃЌ2Fe3ЃЋЃЋFe=3Fe2ЃЋ ,2HЃЋЃЋFe=Fe2ЃЋЃЋH2Ёќ
ЂШЩшMgOЮЊxmol,дђга40g/molx+160g/molx="20g,x=0.1mol," c(Mg2+)=0.1mol/0.15L=0.67mol/L



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК101ЭјаЃЭЌВНСЗЯАЁЁИпвЛЛЏбЇЁЁЩНЖЋПЦбЇММЪѕГіАцЩч ТГНЬАц ЬтаЭЃК022
ЗлФЉзДЪдбљAЪЧгЩЕШЮяжЪСПЕФMgOКЭFe2O3зщГЩЕФЛьКЯЮяЃЌНјааШчЯТЪЕбщЃК
ЂйШЁЪЪСПAНјааТСШШЗДгІЃЌВњЮяжагаЕЅжЪBЩњГЩЃЛ
ЂкСэШЁ20 gAШЋВПШмгк0.15 LЁЁ6.0 mol/LЕФбЮЫсжаЕУШмвКCЃЛ
ЂлНЋЂйжаЕУЕНЕФЕЅжЪBКЭШмвКCЗДгІЃЌЗХГі1.12 LЦјЬх(БъПі)ЃЌЭЌЪБЩњГЩШмвКDЃЌЛЙВаСєгаЙЬЬхЮяжЪBЃЛ
ЂмгУKSCNШмвКМьбщЪБШмвКDВЛБфЩЋЃЎ
ЧыЬюПеЃК
(1)Ђйжав§ЗЂТСШШЗДгІЕФЪЕбщВйзїЪЧ________ЃЌВњЮяжаЕФЕЅжЪBЪЧ________ЃЎ
(2)ЂкжаЫљЗЂЩњЕФИїЗДгІЕФЛЏбЇЗНГЬЪНЪЧ________ЃЎ
(3)ЂлжаЫљЗЂЩњЕФИїЗДгІЕФРызгЗНГЬЪНЪЧ________ЃЎ
(4)ШєШмвКDЕФЬхЛ§ШдЪгЮЊ0.15 LдђИУШмвКжаc(Mg2+)ЮЊ________ЃЌc(Fe2+)ЮЊ________ЃЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК101ЭјаЃЭЌВНСЗЯАЁЁИпвЛЛЏбЇЁЁЩНЖЋПЦбЇММЪѕГіАцЩч ТГНЬАц ЬтаЭЃК038
ЗлФЉзДЪдбљAЪЧгЩЕШЮяжЪСПЕФMgOКЭFe2O3зщГЩЕФЛьКЯЮяЃЌНјааШчЯТЪЕбщЃК
ЂйШЁЪЪСПAНјааТСШШЗДгІЃЌВњЮяжагаЕЅжЪBЩњГЩЃЛ
ЂкСэШЁ20 gAШЋВПШмгк0.15 LЁЁ6.0 mol/LЕФбЮЫсжаЕУШмвКCЃЛ
ЂлНЋЂйжаЕУЕНЕФЕЅжЪBКЭШмвКCЗДгІЃЌЗХГі1.12LЦјЬх(БъПі)ЃЌЭЌЪБЩњГЩШмвКDЃЌЛЙВаСєгаЙЬЬхЮяжЪBЃЛ
ЂмгУKSCNШмвКМьбщЪБШмвКDВЛБфЩЋЃЎ
ЧыЬюПеЃК
(1)Ђйжав§ЗЂТСШШЗДгІЕФЪЕбщВйзїЪЧ________ЃЌВњЮяжаЕФЕЅжЪBЪЧ________ЃЎ
(2)ЂкжаЫљЗЂЩњЕФИїЗДгІЕФЛЏбЇЗНГЬЪНЪЧ________________ЃЎ
(3)ЂлжаЫљЗЂЩњЕФИїЗДгІЕФРызгЗНГЬЪНЪЧ________________ЃЎ
(4)ШєШмвКDЕФЬхЛ§ШдЪгЮЊ0.15 LЃЌдђИУШмвКжаc(Mg2+)ЮЊ__________ЃЌc(Fe2+)ЮЊ________ЃЎ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com