
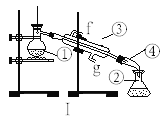
����Ŀ������Ԫ����(Te)�ǵ�����¼����²��ϵ���Ҫ�ɷ�֮һ����֪��TeO2�������������ҵ�ϳ��á�ͭ�����ࡱ(��Ҫ��Cu2Te����������Ag��Au)Ϊԭ���Ʊ������ڣ��乤��������ͼ��ʾ��

�ش��������⣺
(1)�ڣ�Te��λ��Ԫ�����ڱ���_________���ڵ���A�壻�����й�ȷ�����ʵ������������______��
A.�ڵĵ����ڳ������ǹ�̬ B.�ڵij������ϼ���-2��+4��+6
C.�ڿ��������뵼����� D.�ڵ��⻯��H2Te���ȶ�
(2)ʵ�����С�����I����������________�������������ʵ������ص���________��
A.����©�� B.�ձ� C.��ƿ D.������ E.����ƿ
(3)�ڡ���ѹ��������������У�ÿ����1mol Cu2Te��Ӧ����TeOSO4��ת�Ƶĵ�����Ϊ________mol�����������Һ����������Ҫ�ɷֳ�TeOSO4�⣬������________��д��ѧʽ����
(4)����������ͭ��ʱ��������ø����Һ�����ڵ����У�ͭ��ȷ�����Ĺ�ϵ��ͼ��ʾ������ʼ�������ĵ缫��Ӧʽ��__________��

(5)����ȷ����ͭ��Һ����ͨ��SO2��ԭTeOSO4���ɰ��Ļ�ѧ����ʽΪ__________��
��������Ӧ�ڼ�������NaCl��Ӧ���ʴ���ȼӿ죬��NaCl����Ҫ����Ϊ_____________��
���𰸡�5 D ���� ACE 10 CuSO4��H2SO4 Cu2++2e-=Cu TeOSO4+2SO2+3H2O=Te+3H2SO4 ����
��������
�Ʊ�����������Ϊ�����������������ȡ��ͭ��������(��Ҫ��Cu2Te����������Ag��Au)�õ�TeOSO4��H2SO4��CuSO4��Һ��Ag��Au���壬���˵õ�TeOSO4��H2SO4��CuSO4��Һ������ͭ���˵õ���ͭ��TeOSO4��Һ(��������CuSO4)����NaCl��������SO2��ԭTeOSO4���ɴ��ڣ��ݴ˽��
(1)��λ�����ڱ��������ڵڢ�A�壬����ͬ��Ԫ�ص����������Ժ͵ݱ��Է�����
(2) ����I�Ƿ��������Թ������Һ�IJ��������˲�������Ҫ�IJ�������Ϊ�ձ�����������©����
(3)Cu2Te��Te�Ļ��ϼ�Ϊ-2��Cu�Ļ��ϼ�Ϊ+1����Ӧ����CuSO4��TeOSO4ʱ��������Cu2Te��(TeOSO4+2CuSO4)��10e-����ϵ���ͭ��֪�������ڽ���Һ����������Ҫ�ɷ�ΪTeOSO4��H2SO4��CuSO4��
(4)��ͭ���ڳ����Ĺ�ϵͼ��֪������ʼ��Cu2+�����ߣ���Cu�����ʴﵽ13%����ʱ�ڿ�ʼ����������
(5)��SO2��ԭTeOSO4�����ں�H2SO4��
�ڷ�Ӧ�ڼ�������NaCl��Ӧ���ʴ���ȼӿ죬����NaC1����Ҫ����Ϊ������
(1)����52��Ԫ�أ�ԭ�Ӻ�����5�����Ӳ㣬�����6�����ӣ������Ԫ��λ�����ڱ��������ڵڢ�A�壻
A��Te��Sλ��ͬһ���壬���Ԫ�ر仯���ɿ�֪O��S��Se��Te����̬�����������壬�����ڵĵ����ڳ������ǹ�̬��A��ȷ��
B��Te��Sλ��ͬһ���壬��ѧ���������Ƶ㣬SԪ�صij������ϼ���-2��+4��+6������Te�ij������ϼ���-2��+4��+6��B����ȷ��
C����(Te)�ǵ�����¼����²��ϵ���Ҫ�ɷ�֮һ����;������Si�������ڿ��������뵼����ϣ�C��ȷ��
D��S�ķǽ����Դ����ڣ�H2S���ȶ��Բ�ǿ������Te���⻯��H2Te�ȶ��Ա�H2S������H2Te���ȶ���D����
�ʺ���ѡ����D��
(2)���������Թ������Һ�����IJ������������������� ����IΪ���ˣ����˲�������Ҫ�IJ�������Ϊ�ձ�����������©�������Բ���Ҫ����©������ƿ������ƿ��ѡ�������ΪABF��
(3)����ѹ���������������Cu2Te�е�Te��Cu������������Cu2Te��(TeOSO4+2CuSO4)��10e-����������1molCu2Te��Ӧ����TeOSO4ʱ��ת�Ƶĵ�����Ϊ10mol����ϵ���ͭ��֪�������ڽ���Һ����������Ҫ�ɷֻ���H2SO4��CuSO4��
(4)��ͭ���ڳ���ͼ��֪������ʼ��Cu2+�����ߣ�����������Cu2+���������ŵ磬�缫��ӦʽΪCu2++2e-=Cu��
(5)��SO2��ԭTeOSO4�����ں�H2SO4�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�Ļ�ѧ����ʽΪ2SO2+3H2O+TeOSO4=Te+3H2SO4��
�ڷ�Ӧ�ڼ�������NaCl��Ӧ���ʴ���ȼӿ죬����NaCl�����뷴Ӧ��˵��NaC1����Ҫ����Ӧ��Ϊ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����______ molAl2(SO4)3�к�0.3 mol SO42�C����Al3+Լ_______________����
��2��ͬ��ͬѹ�£���ͬ������SO2�����SO3���壬�������֮��Ϊ_________���ܶ�֮��Ϊ_________��
��3������ͬΪ46 g���������壬NO2��N2O4�����ʵ���֮��Ϊ________����������Oԭ�Ӹ���֮��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ����ʳ���Ϳɻ��ij�����л�������X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
��1��X�ķ���ʽ��________��
��2��X������Ʒ�Ӧ�ų���������Ӧ�Ļ�ѧ����ʽ��____________(�л����ýṹ��ʽ����)��
��3��X������е�������ͭ�����������·�Ӧ����Y��Y�Ľṹ��ʽ��________��
��4��X��������������Һ��Ӧ������Z��Z������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[���ʽṹ������һѡ��3]
������̼��Ԫ�صĵ��ʼ��仯���������Ҫ���о���ֵ��
(1)��(Ge)�ĺ���ʮ��ϡ�٣��������㷺Ӧ���ڵ��ӡ���ѧ������������ҽѧ����Դ���������¿Ƽ�����
���ִ���ѧ�У�������__________�ϵ���������������Ԫ�ء���̬��ԭ���У������ܼ���ߵ�ԭ�ӹ���ϣ����еĵ�����Ϊ___________��
��ͨ��״����GeCl4����ɫҺ�壬�ӷ������۵�Ϊ-51.50C���е�Ϊ86.55�������������ѡ��ɴ˿���֪GeCl4Ӧ���ڹ��ۻ������������_______��Ҳ���Ⱥ�����Ԫ�صĵ縺�����Ӧ��_______1.7(����С��������������)��
(2)�ڹ������У�![]() ��ͼa��ʾΪ������ṹ��������ͨ�����ö���0ԭ�ӣ��γ���״��״����״����״�ṹ�IJ�ͬ�����Ρ�ͼb��ʾ��n�����������ӳɵĹ����������Si���ӻ���ʽΪ_____��Si��O��ԭ�Ӹ�����Ϊ________����ѧʽ�ɱ�ʾΪ__________��
��ͼa��ʾΪ������ṹ��������ͨ�����ö���0ԭ�ӣ��γ���״��״����״����״�ṹ�IJ�ͬ�����Ρ�ͼb��ʾ��n�����������ӳɵĹ����������Si���ӻ���ʽΪ_____��Si��O��ԭ�Ӹ�����Ϊ________����ѧʽ�ɱ�ʾΪ__________��

(3)ʯī�����ɲ�״ʯī����������ABAB��ʽ�ѻ����ɣ���ͼ(a)��ʾ��ͼ(b)��һ��ʯī����������ʾ��ͼ��

������ͼ �л���������c���ͶӰ (�����������̼ԭ��λ�ü���)_________��
�л���������c���ͶӰ (�����������̼ԭ��λ�ü���)_________��
������ʯī������Ϊh cm��C-C����Ϊr cm����ʯī�����ܶȵı���ʽΪ__________g.cm-3(�����ӵ�����ΪNA)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С���ú���Ϊԭ����ȡ��������ˮ������CCl4�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ����ʵ������ɷֽ�Ϊ���¼�����
A����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
B����50mL��ˮ��15mLCCl4�����Һ©���У����Ǻò�������
C�������Һ©���������ϿڵIJ������Ƿ�©Һ��
D����ת©������������ʱ�����������������رջ������ѷ�Һ©��������
E���������������ձ������²�Һ�壻
F���ӷ�Һ©���ڵ����ϲ�ˮ��Һ��
G����©���Ͽڲ�������ʹ���ϵİ��ۻ�С��©���ϵ�С�ף�
H�����á��ֲ㡣
��1������E����IJ�����Ӧע�⣺________��
��2������G����IJ�����Ŀ���ǣ�________��
��3������CCl4�ӵ�ˮ����ȡ���ԭ����_____��
��4��������װ��I�������Ȼ�̼�͵�Ļ�����ȱ�ٵ�������______��������������������е�ʵ�����������Ϊ��_____�������ۺܵ͢����ƣ���______��______����������250ml0.2 mol/LNaCl��Һ��װ��II��ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����_______ ��________

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10��ʱij��ѧ��Ӧ����Ϊ0.1mol/ (L��s)�����¶�ÿ����10����Ӧ�������ӵ�ԭ����2����Ϊ�˰Ѹ÷�Ӧ������ߵ�1.6 mol/(L��s)����÷�Ӧ����ʲô�¶��½���
A. 30�� B. 40�� C. 50�� D. 60��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ϊ2L������ܱ������м���1 mol CaCO3��������Ӧ��CaCO3(s) ![]() CaO(s)+CO2(g)����ö�����̼�����ʵ���Ũ�����¶ȵı仯��ͼ��ʾ��ͼ������A��ʾCO2��ƽ��Ũ�����¶ȵĹ�ϵ��B�Dz�ͬ�¶��£���Ӧ������ͬ��ʱ��ʱ��CO2���ʵ���Ũ�ȵı仯����.
CaO(s)+CO2(g)����ö�����̼�����ʵ���Ũ�����¶ȵı仯��ͼ��ʾ��ͼ������A��ʾCO2��ƽ��Ũ�����¶ȵĹ�ϵ��B�Dz�ͬ�¶��£���Ӧ������ͬ��ʱ��ʱ��CO2���ʵ���Ũ�ȵı仯����.

��ش��������⣺
��1����֪����CaO(s)+SO2(g)==CaSO3(s) ��H1=��402kJ��mol��1
��2CaCO3(s)+2SO2(g)+O2(g)==2CaSO4(s)+2CO2(g) ��H2=��2762kJ��mol��1
��2CaSO3(s)+O2(g)==2CaSO4(s) ��H3=��2315kJ��mol��1
��CaCO3(s)==CaO(s)+CO2(g)����H=____kJ��mol��1
��2�����¶�ΪT5��ʱ����ӦCaCO3(s) ![]() CaO(s)ʮCO2(g)��ʱ20s�ﵽƽ�⣬��20s�ڸ÷�Ӧ�ķ�Ӧ����Ϊv(CO2)=____����Ӧ��ƽ�ⳣ��Ϊ____mol��L��1��
CaO(s)ʮCO2(g)��ʱ20s�ﵽƽ�⣬��20s�ڸ÷�Ӧ�ķ�Ӧ����Ϊv(CO2)=____����Ӧ��ƽ�ⳣ��Ϊ____mol��L��1��
������÷�Ӧ��ƽ�ⳣ��Kֵ��÷�Ӧ____(ѡ����)��
A.һ�����淴Ӧ�����ƶ� B.��ƽ���ƶ�ʱ����Ӧ������������С
C.һ��������Ӧ�����ƶ� D.��ƽ���ƶ�ʱ�淴Ӧ�����ȼ�С������
��3����T5���£�ά���¶Ⱥ�����������䣬����CO2���壬��ﵽƽ��ʱCaCO3������____(����������������С������������)��
��4����T5���£���Ӧ�ﵽƽ��״̬����ѹ������Ϊ1L�����´ﵽƽ��ʱ��CO2��Ũ��____(����������������С������������)
��5�������¶ȵ����ߣ�����B������A�ƽ���ԭ����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Т�0.112Lˮ����3.01��1023���Ȼ�����ӣ���13.6 g H2S���壻��0.2 mol���������ж����������ʵĹ�ϵ��С����������ȷ���ǣ� ��
A. ���������٢ۢڢ�B. �ܶȣ��ܢ٢ۢ�
C. �����٢ܢ�D. ��ԭ�������ڢܢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ���ӹ�����ֻ���ڷ��Ӽ���������������������ѧ��
B. �γɹ��ۼ���Ԫ��һ���Ƿǽ���Ԫ��
C. ���ۼ��ı�������ԭ�ӹ�����ص��̶��й�
D. �� �����Ե����γ��ҿ����Ƽ�����ת���� ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com