
(8��)��֪��CH3CH2OH+NaBr+H2SO4(Ũ)  CH3CH2Br+NaHSO4 +H2O��
CH3CH2Br+NaHSO4 +H2O��
ʵ�����Ʊ������飨�е�Ϊ38.4�棩��װ�úͲ������£�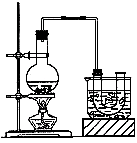
�ٰ���ͼ��ʾ�������������װ�õ������ԣ�Ȼ����U�ιܺʹ��ձ�������ˮ������Բ����ƿ�м���10mL95���Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ����С����ȣ�ʹ���ַ�Ӧ��
�Իش��������⣺
(1)��Ӧʱ���¶ȹ��߿ɿ����к���ɫ���������������Ļ�ѧʽΪ
��
��2��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
(3)��Ӧ������U�ι��д��Ƶ���������ػ�ɫ����U�ι��еĻ���ﵹ���Һ©���У����ã���Һ��ֲ��Һ��ȡ (��ϲ㡱���²㡱)Һ�塣Ϊ�˳�ȥ���е����ʣ���ѡ�������Լ��е� (�����)��
| A��Na2SO3��Һ | B��H2O | C��NaOH��Һ | D��CCl4 |
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
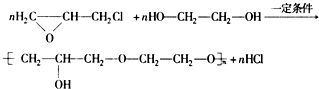
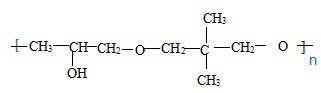
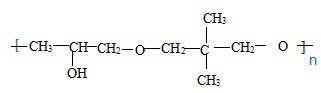
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
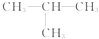 ��
��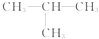 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

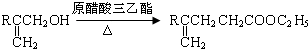


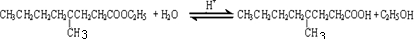
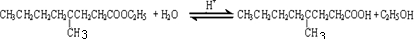
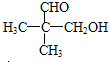
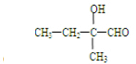
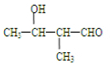
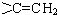 ��ͬ���칹����
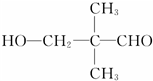
��ͬ���칹���� ���˴Ź���������3���壬�ҷ����֮��Ϊ9��2��1��ͬ���칹��Ľṹ��ʽΪ
���˴Ź���������3���壬�ҷ����֮��Ϊ9��2��1��ͬ���칹��Ľṹ��ʽΪ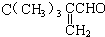
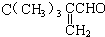
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
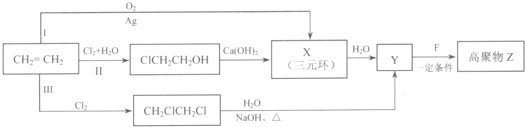

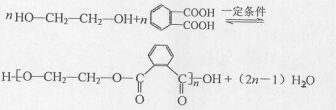

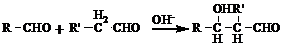 ��R��R'������������ԭ�ӣ�
��R��R'������������ԭ�ӣ�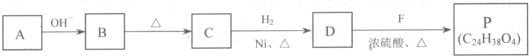
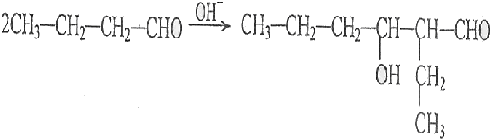
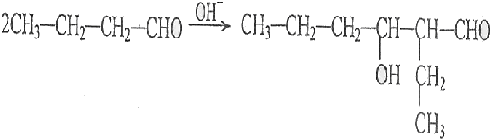
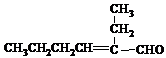
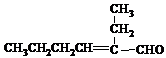
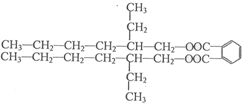

| �Ǿ���Co-B�Ͻ� |
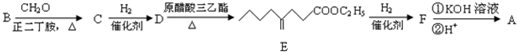
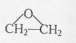
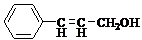 ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�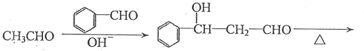
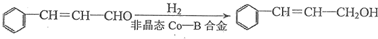

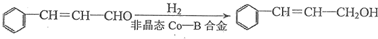
| �Լ�1 |
| ����1 |
| �Լ�2 |
| ����2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ��
��
 ��
��
| �� |
| �� |



�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com