
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�á�

��1��װ��B����֮һ��Ϊ�˳�ȥ�����е�����HCl��ʢװ��Һ���Լ�Ϊ__________��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η����������ȷ����__________ (����ĸ���) ��
��� | �� | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��3��D�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________����װ��D�е���Һ����װ��E�У���Һ��Ϊ���㣬�ϲ���Ϻ�ɫ��Ҫ������Ϻ�ɫ��Һ����ʹ�õIJ��������Dz��������ձ���_____________��
��4��װ��F�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
��5�������ʵ��ʹ�õ�Ũ������������Ϊ36.5%���ܶ�Ϊ1.15 g/cm3������������ʵ���Ũ��Ϊ_____mol��L-1������250 mLˮ���ƣ���Ӧ�ܽ�����HCl����ԼΪ_____________L���������3λ��Ч���֣���
���𰸡�����ʳ��ˮ d Cl2+2NaBr=2NaCl+Br2 ��Һ©�� Cl2+2NaOH=NaCl+NaClO+H2O 11.5 88.2
��������
(1)����ʳ��ˮ�����������е�HCl��(2)Ϊ����֤�����Ƿ����Ư���ԣ�I�м���ʪ�����ɫ������IIΪU�ܣ��ɼ�������������õ������Cl2��III�м���������ɫ����������֤��Cl2�Ƿ����Ư���ԣ�(3) D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����Ȼ��ƺ��嵥�ʣ�������ӦCl2+2NaBr=Br2+2NaCl���÷�Һ�����뻥�����ݵ�Һ������4��װ��F�����������������������Ȼ�����������������5������![]() ������������ʵ���Ũ�ȣ�����
������������ʵ���Ũ�ȣ�����![]() ��������HCl����������
��������HCl����������
(1)����ʳ��ˮ�����������е�HCl��װ��B�е��Լ��DZ���ʳ��ˮ����2��Ϊ����֤�����Ƿ����Ư���ԣ�I�м���ʪ�����ɫ������IIΪU�ܣ��ɼ�������������õ������Cl2��III�м���������ɫ����������֤��Cl2�Ƿ����Ư���ԣ���ѡd��(3) D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����Ȼ��ƺ��嵥�ʣ�������ӦCl2+2NaBr=Br2+2NaCl���÷�Һ�����뻥�����ݵ�Һ����Ҫ������Ϻ�ɫ��Һ����ʹ�õIJ��������Dz��������ձ��ͷ�Һ©������4��װ��F�����������������������Ȼ�����������������Ӧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O����5��![]() =11.5 mol��L-1������Ҫ�����HCl��������ΪVL����HCl�����ʵ�����
=11.5 mol��L-1������Ҫ�����HCl��������ΪVL����HCl�����ʵ�����![]() ����Һ��������
����Һ��������![]() ����Һ�����
����Һ�����![]() ������
������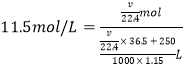 ����V= 88.2L��
����V= 88.2L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С�����Fe(OH)3������Ʊ�ʵ�鲢���������ʡ�
(1)��������FeCl3��Һ�ֱ��������Һ���У����γɽ������________(����ĸ)��
A����ˮ B����ˮ C��NaOHŨ��Һ D��NaClŨ��Һ
(2)д���Ʊ�Fe(OH)3����Ļ�ѧ����ʽ____________________________��
(3)����������������ֱ���ķ�Χ��______��֤�������������������ɵ�ʵ�������______________��
(4)ȡ�����ƵõĽ�������Թ��У�����(NH4)2SO4��Һ��������_________�����������Ϊ�����________��
(5)�������������ȶ����ڵ���Ҫԭ����________(����ĸ����ͬ)��
A��������ֱ��С��1 nm B���������������
C���������������˶� D��������������ֽ
(6)Fe(OH)3����������FeCl3��Һ�����������________��
A��Fe(OH)3��������ֱ����1��100 nm֮��
B��Fe(OH)3�������������
C��Fe(OH)3�����Ǿ�һ�ķ�ɢϵ
D��Fe(OH)3����ķ�ɢ��������ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4(g) ![]() 2NO2(g)�����¶����ߣ�����������ɫ���
2NO2(g)�����¶����ߣ�����������ɫ���
��1����Ӧ����H________0����������������С��������100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L-1��s-1����Ӧ��ƽ�ⳣ��K1Ϊ_____________��

��2��100 ��ʱ�ﵽƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.0020 mol��L-1��s-1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣T______100 ������������������С���������ж�������____________________________________________��
��3���¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________����������Ӧ�������淴Ӧ���������ƶ����ж�������____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Cu2O��һ�ְ뵼����ϣ�������ɫ��ѧ������Ƶ���ȡCu2O�ĵ���ʾ��ͼ���ң�����ܷ�ӦΪ��2Cu��H2O![]() Cu2O��H2��������˵����ȷ����
Cu2O��H2��������˵����ȷ����

A. ʯī�缫�ϲ�������
B. ͭ�缫������ԭ��Ӧ
C. ͭ�缫��ֱ����Դ�ĸ���
D. ����0.1 mol����ת��ʱ����0.1 mol Cu2O����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ת��������ú��ʯ�͵��ۺ����õ��ǣ���
A.��ú�����Ƶ�ú���ͺͽ�̿
B.��һ�������½�ú������ת��ΪҺ��ȼ��
C.��ʯ���ѽ��Ƶ���ϩ�Ȼ���ԭ��
D.��ú��Ϊú����ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ��һ�ַ���ʽΪC4H8O2���л���ĺ������ͼ������л������Ϊ�� ��
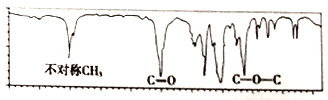
A. HCOOCH2CH2CH3 B. CH3CH2COOH
C. CH3COOCH2CH3 D. (CH3)2CHCH2COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ż��жԡ��Bʯ�������¼��أ����Bʯ����Ȼ֮ͭ��Ҳ����¯��ʯ(��Ҫ�ɷ�Ϊ̼��п)������,���BҲ���ޕPԻ:ͭһ�¯��ʯһ��,��֮���Bʯ(���B)������ˮ���أ��Bʯ�����ϡ������������������
A. ���B��һ�ֺϽ�
B. ¯��ʯ�е�̼��п��ұ���б���ԭ
C. ���������������١��Bʯ��
D. �ֱ�����Ǻ͡��Bʯ��������ˮ���ء���ԭ������ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������480 mL 1 mol�� L��1��ϡ���ᡣ�ɹ�ѡ�õ������У��� ��ͷ�ιܢ� ��ƿ�� �ձ��� �������� ҩ�ע� ��Ͳ�� ������ƽ����ش��������⣺

��1��������ƿ��ʹ�÷����У����в�������ȷ����________
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ����������Һ����Ҫ����
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������������ˮ���ӽ�����1��2cm�����õιܼ�����ˮ������
D�����ݺ�Ǻ�ƿ����ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ�ߵ�ҡ�ȶ��
��2����Ũ��������ʵ���Ũ��Ϊ_________mol�� L��1��
��3������ϡ����ʱ����ȱ�ٵ�������________________��
��4������480mL 1mol�� L��1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ_______mL��������Ͳ�Ĺ��Ϊ________�����ɹ�ѡ�����Ͳ��5mL��10mL��20mL��50mL��100mL��
��5�������еIJ��ֲ��裬�����д������(��д���)____________��

��6���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol�� L��1�����ƹ��������и���������������Ũ��ƫ�ߵ�ԭ����___________��
A������ʱ����������ƿ�̶��߽��ж��ݡ�
B����ϡ�ͺ����������ת������ƿ�����žͽ����Ժ��ʵ�������
C������ƿ������ˮϴ�Ӻ�δ���������������ˮ��
D��ת����Һʱ��������������Һ��������ƿ���档
E�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������Ҫԭ�����ŷ�SO2�йص���( )
A.����
B.�⻯ѧ����
C.�����ն�
D.����ЧӦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com