
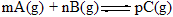 ����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______��
����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪���淴Ӧ��FeO��s��+CO��g��
��֪���淴Ӧ��FeO��s��+CO��g�� Fe��s��+CO2��g����������ҵ�е�һ����Ҫ��Ӧ�����¶�T��n��CO2��/n��CO���ı�ֵ��ϵ�����±���ʾ�������й�˵����ȷ���ǣ�������
Fe��s��+CO2��g����������ҵ�е�һ����Ҫ��Ӧ�����¶�T��n��CO2��/n��CO���ı�ֵ��ϵ�����±���ʾ�������й�˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ����NH3�����ʵ��� |
| y | NH3�����ʵ��� | ƽ�ⳣ��K | NH3��ת���� | ���������ʵ����ܺ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ̩����ѧ������ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��ӦCO(g)+2H2(g)  2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
| ����� | 1 | 2 | 3 | 4 | |
| ��Ӧ�¶�/�� | 225 | 235 | 225 | 235 | |
| ��Ӧǰ��������ʵ���/mol | CO2 | 0 | 0 | 0.2 | 0.2 |
| CO | 3.0 | 3.0 | 2.8 | 2.8 | |
| H2 | 7.0 | 7.0 | 7.0 | 7.0 | |
| ƽ��ʱCH3OH���������/�� | 4.9 | 8.8 | 36.5 | 50.7 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ����12���¿���ѧ�Ծ��������棩 ���ͣ������
�����ѣ�CH3OCH3������ɫ���壬����Ϊһ��������Դ���ɺϳ��������ΪH2��CO��������CO2��ֱ���Ʊ������ѣ�������Ҫ���̰��������ĸ���Ӧ����Ϊ���淴Ӧ����
��CO(g)+ 2H2(g) = CH3OH(g) ��H1=��90��1 kJ��mol-1
��CO2(g)+ 3H2(g) = CH3OH(g)+H2O(g) ��H2=��49��0 kJ��mol-1
ˮú���任��Ӧ ��CO(g) + H2O (g)=CO2(g)+H2(g) ��H3=��41��1 kJ��mol-1
�����Ѻϳɷ�Ӧ ��2CH3OH(g)=CH3OCH3(g)+H2O(g) ��H4=��24��5 kJ��mol-1
��1����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ ��
��2��һ���¶��£��ں����ܱ������н��з�Ӧ�٣�����������˵����Ӧ����ƽ��״̬���� ��
a.����������ƽ����Է����������ֲ���
b.�����������ܶȱ��ֲ���
c.CH3OH(g)Ũ�ȱ��ֲ���
d.CH3OH(g)���������ʵ���H2 (g)����������
��3��һ���¶��£���8mol CH3OH(g)����5L�ܱ������н��з�Ӧ�ܣ�һ��ʱ���ƽ��״̬����Ӧ�����й��ų�49kJ��������CH3OH(g)��ƽ��ת����Ϊ �����¶��£�ƽ�ⳣ��K= �����¶��£����������ٳ���2mol CH3OH(g)�����ٴδﵽ��ƽ��״̬���ж���ȷ���� ��
a.CH3OH(g)��ƽ��ת���ʼ�С
b.CH3OCH3 (g)�������������
c.H2O(g)Ũ��Ϊ0��5mol��L-1
d.�����е�ѹǿ��Ϊԭ����1��25��
��4�������ѡ�����ȼ�ϵ�ؾ��������죬Ч�ʸߵ��ŵ㣬�������ܶȸ��ڼ״�ȼ�ϵ�أ��������Ϊ���ԣ������ѡ�����ȼ�ϵ�صĸ�����ӦΪ ������2��8L(��״��)����ʱ���������������·�ĵ��� mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ӦCO(g)+2H2(g)  2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
|
����� |
1 |
2 |
3 |
4 |
|
|
��Ӧ�¶�/�� |
225 |
235 |
225 |
235 |
|
|
��Ӧǰ��������ʵ���/mol |
CO2 |
0 |
0 |
0.2 |
0.2 |
|
CO |
3.0 |
3.0 |
2.8 |
2.8 |
|
|
H2 |
7.0 |
7.0 |
7.0 |
7.0 |
|
|
ƽ��ʱCH3OH���������/�� |
4.9 |
8.8 |
36.5 |
50.7 |
A���÷�Ӧ�ġ�H>0
B����������ѹǿ����ʱ����Ӧ�ﵽƽ��
C��CH3OH���������ԭ����CO2���˴�����
D������CO2�����ƽ��ʱCH3OH���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com