

���� ��1��Cuԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��ʧȥ4s�ܼ�1�������γ�Cu+���ݴ��жϵ���ռ�ݵ�ԭ�ӹ����Ŀ��
��2�����ݺ��ع����������ͬһ�ܲ��Ͼ�����ռ�ݲ�ͬ�Ĺ�����ݴ˴��⣻
��3��ͬ�������϶��µ�һ�����ܼ�С��PԪ��3p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ���Si�ĵ�һ��������С��Pԭ�ӵ��ĵ�����Ϊʧȥ4s2����1�����ӣ�Ϊȫ���ȶ�״̬����������������ϴ�
��4���ڶ������辧�壬���Կ����ھ������ÿ��Si-Si��֮������Oԭ�ӣ�Si��Oԭ�������ӵ���С��Ϊ12Ԫ��������Si��ÿ��Siԭ���γ�4��Si-Si������ͼ2��֪ÿ��Si-O-Si�������γ�6��12Ԫ����
��5������ͼ3��֪��Na2[B4O5��OH��4]8H2O�д������Ӽ������ۼ������»����������������Ϊ[B4O5��OH��4]2-������ͨ����������γ���״�ṹ���ݴ˴��⣻
��6������GaN�ľ���ṹͼ��֪��ÿ����ԭ�ӻ�ÿ����ԭ����Χ���γ��ĸ����ۼ����������ǵ��ӻ���ʽ����sp3��������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
��7�����ݾ�̯�����㾧����Ge��Oԭ����Ŀ����������ԭ��������ʾ�����������ٽ��m=��V���㣮
��� �⣺��1��Cuԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��ʧȥ4s�ܼ�1�������γ�Cu+�������ռ�ݵ�14ԭ�ӹ����
�ʴ�Ϊ��14��
��2�����ݺ��ع����������ͬһ�ܲ��Ͼ�����ռ�ݲ�ͬ�Ĺ�������Ի�̬��ԭ�Ӽ۲�����Ų�ʽд��4s24px24py4��Υ���˺��ع���
�ʴ�Ϊ�����ع���
��3��ͬ�������϶��µ�һ�����ܼ�С��PԪ��3p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ���Si�ĵ�һ��������С����ͼ�е�һ�����ܿ�֪��cΪSi��Pԭ�ӵ��ĵ�����Ϊʧȥ4s2����1�����ӣ�Ϊȫ���ȶ�״̬����������������ϴ�֪bΪP��aΪC��
�ʴ�Ϊ��b��
��4���ڶ������辧�壬���Կ����ھ������ÿ��Si-Si��֮������Oԭ�ӣ�Si��Oԭ�������ӵ���С��Ϊ12Ԫ��������Si��ÿ��Siԭ���γ�4��Si-Si������ͼ2��֪ÿ��Si-O-Si�������γ�6��12Ԫ����
�ʴ�Ϊ��12��6��
��5������ͼ3��֪��Na2[B4O5��OH��4]8H2O�д������Ӽ������ۼ������»���������������ڽ���������ѡC��������Ϊ[B4O5��OH��4]2-������ͨ����������γ���״�ṹ��
�ʴ�Ϊ��C�������
��6������GaN�ľ���ṹͼ��֪��ÿ����ԭ�ӻ�ÿ����ԭ����Χ���γ��ĸ����ۼ����������ǵ��ӻ���ʽ����sp3��������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
�ʴ�Ϊ���ǣ�������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
��7��������Geԭ����ĿΪ4��Oԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ѧʽΪGeO����������ԭ������ΪM����;�������Ϊ��$\frac{4����M+16��}{6.02��10{\;}^{23}}$g����$\frac{4����M+16��}{6.02��10{\;}^{23}}$g=7.4g��cm-3����4.3��l0-8 cm��3�����M=72.5��
�ʴ�Ϊ��GeO��72.5��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ������ṹ����㡢�����ӻ�����ȣ���3��ע����ݺ��ع���������������ܾ������⣬��4��Ϊ�״��㣬��Ҫѧ���߱�һ���Ŀռ�������۲�������


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�1L �״���ȫȼ�պ����ɵ� CO2 ���Ӹ���ԼΪ$\frac{1}{2.42}$N A | |
| B�� | N A��H2���ӵ�����ԼΪ2g������ռ�����ԼΪ 22.4L | |
| C�� | ��״���£�22.4L CO2��CO�Ļ�������к��е�̼ԭ����ΪN A | |
| D�� | 500mL 0.5mol/L ��Ca��ClO��2��Һ�У�����ClO-����ĿΪ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 X��Y��Z��W��R��QΪǰ30��Ԫ�أ���ԭ��������������X������Ԫ����ԭ�Ӱ뾶��С�ģ�Y�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Zԭ�ӵ���������ͬ����Ԫ������࣬W��Zͬ���ڣ���һ�����ܱ�Z�ĵͣ�R��Yͬһ���壬Q�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬����ش��������⣺
X��Y��Z��W��R��QΪǰ30��Ԫ�أ���ԭ��������������X������Ԫ����ԭ�Ӱ뾶��С�ģ�Y�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Zԭ�ӵ���������ͬ����Ԫ������࣬W��Zͬ���ڣ���һ�����ܱ�Z�ĵͣ�R��Yͬһ���壬Q�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �մɡ�ˮ�ࡢʯӢ���������ά�����ڹ����β�Ʒ | |
| B�� | ú����õ���ú���ͣ�����Ҫ��;�Ǿ�����õ�ȼ�ͣ���һӦ�ÿ����֡���̼��������� | |
| C�� | �����������Ի�ѧҩƷ���У��Ҵ������ࡢ����������Һ��˫��ˮ�����Խ������������ﵽ������Ŀ�� | |
| D�� | ɨ�����������������ֱ�ӫ���������ȿƼ��ֶεķ�չ���ٽ���������۽ṹ��̽������ʵ�ֶ�ԭ�ӻ���ӵIJٿأ�ʹ��ѧ�о��������ˮƽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Al3+��Cl-��CO32- | B�� | Na+��Fe3+��Cl-��NO3- | ||
| C�� | H+��Na+��ClO-��S2- | D�� | K+��NH4+��OH-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
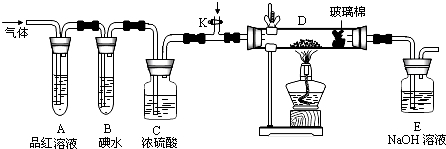
�谢���ӵ�������ֵΪNA����״����O2��N2�Ļ������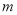 g����b�����ӣ���
g����b�����ӣ��� g�û����������ͬ״������ռ�����(L)Ӧ��
g�û����������ͬ״������ռ�����(L)Ӧ��
A��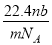 B��
B�� C��
C��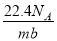 D��
D��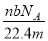
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com