
| ������ | Ba2+ NH4+ Fe3+ Al3+ Fe2+ |
| ������ | OH- CO32- Cl- SO32- SO42- |
 ���������⡣
���������⡣ �����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ ��
�����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ ��  ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�����ݷ�Ӧ ��֪��������KMnO4��Һ�ɼ����̷� ��֪��������KMnO4��Һ�ɼ����̷� �Ƿ���� �Ƿ���� |
B�����ݷ�Ӧ ��֪����Ӧ��HNO3�������Ժ������� ��֪����Ӧ��HNO3�������Ժ������� |
C�����ݷ�Ӧ ��֪�� ��֪�� �����Ա� �����Ա� ǿ ǿ |
D�����ݷ�Ӧ ��֪�� ��֪��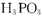 ������Ԫ�� ������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��KCl | B��KNO3 | C��Na2S | D��CuO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢� | C���٢ڢۢ� | D���ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��14Cu2++5S22��+12H2O=7Cu2S+3SO42��+24H+ |
| B��14Cu2++5FeS2+28OH��=7Cu2S+3SO42��+5Fe2++14H2O |
| C��14Cu2+ +5FeS2+24OH��=7Cu2S+3SO42��+5Fe2++12H2O |
| D��14Cu2++5FeS2+12H2O=7Cu2S+3SO42��+5Fe2++24H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 С�Թܸ�
С�Թܸ�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2+O2 | B��CO+CuO | C��Zn+H2SO4 | D��NaOH+CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����SO2����ͨ��NaCIO��Һ�У�SO2+2ClO��+H2O=SO32-+2HCIO | |
| B����FeBr2��Һ��ͨ�����Cl2��2Fe2++4Br��+2Cl2=2Fe3++2Br2+4Cl�� | |
| C�������������Һ�м���Ba��OH��2��Һ�����ԣ�2H++SO42��+Ba2++2OH��=BaSO4��+2H2O�߿� | D��NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH��=NH3��+H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com