
��֪Cu��HNO3���Է������·�Ӧ��
Cu + 4HNO3(Ũ)= Cu(NO3)2 + 2NO2��+ 2H2O��3Cu + 8HNO3(ϡ)=3 Cu(NO3)2 + 2NO��+ 4H2O
��֪22.4gͭ��140mLһ��Ũ�ȵ�����ǡ����ȫ��Ӧ��������NO��NO2��������ڱ�״���µ����Ϊ11.2L������
��1����״����NO��NO2������ֱ��Ƕ��٣�
��2��������������ȫ���ͷź�����Һ�м���2mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת��Ϊ����������NaOH��Һ������Ƕ��٣�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
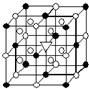 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
| 80m-135n |
| 18n |
| 80m-135n |
| 18n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 I������ʵ����ơ�������ʵ�������������
I������ʵ����ơ�������ʵ�������������| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�����ͼ��ʾ��װ�ý�����ȡNOʵ�飨��֪Cu��HNO3�ķ�Ӧ�Ƿ��ȷ�Ӧ����
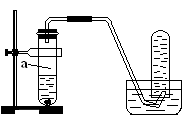
��1���ڼ��װ�õ������Ժ����Թ�a�м���10ml 6 mol��l-1ϡHNO3��1gCuƬ��Ȼ�������ô����ܵ���Ƥ�������Թܿڡ���д��Cu��ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________________________________________________��
��2��ʵ�������ͨ���ڿ�ʼ��Ӧʱ��Ӧ���ʻ���������ӿ죬��������
_________________________________________________;����һ��ʱ�������
��������ԭ����______________________________________________��
��3�����Ͽ���Ƶ�NO���ɲ�ȡ�Ĵ�ʩ��_____________��
A�� ���� B��ʹ��ͭ�� C��ϡ��HNO3 D������ŨHNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���������ϴ����и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��8�֣�����ͼ��ʾ��װ�ý�����ȡNOʵ�飨��֪Cu��HNO3�ķ�Ӧ�Ƿ��ȷ�Ӧ����
��1���ڼ��װ�õ������Ժ����Թ�a�м���10ml 6 mol��l-1ϡHNO3��1gCuƬ��Ȼ�������ô����ܵ���Ƥ�������Թܿڡ���д��Cu��ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________________________________________________��
��2��ʵ�������ͨ���ڿ�ʼ��Ӧʱ��Ӧ���ʻ���������ӿ죬��������
_________________________________________________; ����һ��ʱ�������
��������ԭ����______________________________________________��
��3�����Ͽ���Ƶ�NO���ɲ�ȡ�Ĵ�ʩ��_____________��
| A������ | B��ʹ��ͭ�� | C��ϡ��HNO3 | D������ŨHNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��8�֣�����ͼ��ʾ��װ�ý�����ȡNOʵ�飨��֪Cu��HNO3�ķ�Ӧ�Ƿ��ȷ�Ӧ����
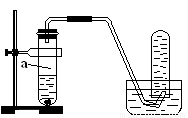
��1���ڼ��װ�õ������Ժ����Թ�a�м���10ml 6 mol��l-1ϡHNO3��1gCuƬ��Ȼ�������ô����ܵ���Ƥ�������Թܿڡ���д��Cu��ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________________________________________________��
��2��ʵ�������ͨ���ڿ�ʼ��Ӧʱ��Ӧ���ʻ���������ӿ죬��������
_________________________________________________; ����һ��ʱ�������
��������ԭ����______________________________________________��
��3�����Ͽ���Ƶ�NO���ɲ�ȡ�Ĵ�ʩ��_____________��
A�� ���� B��ʹ��ͭ�� C��ϡ��HNO3 D������ŨHNO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com