
| ѡ�� | ʵ�鷽�� | ʵ��Ŀ�Ļ���� |
| A | ȡһ��Сľ�������뱥��������Һ�У������ʪ������ȡ�������ɺ����ھƾ������洦��ľ��δȼ�� | ֤�������ƿ���ľ�ķ���� |
| B | ��ȡ��δ֪Ũ������������Һ����ƿ�м���2mL��̪��Ȼ�������ȷ�ζ������һ��������룬��Һ�ɺ�ɫ��Ϊ��ɫ�Ұ���Ӳ��ָ� | ȷ�ж���֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һ�ĵζ����յ� |
| C | ��װ��ʯ��ʯ�ļ������շ������м���Ũ���ᣬ��������������ͨ�뱥��̼��������Һ����ͨ�뱽������Һ�У���������Һ�������� | ���ԣ����̼����� |
| D | ��ʢ�б��ӵ�Ũ��Һ���Թ�����μ���ϡ��ˮ���ߵα��� | ���ӵĶ��Լ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��Ӧ�ü�һ��ľ��������ˮ�ĶԱ�ʵ�飻
B����̪���뼸�μ��ɣ�
C�������ӷ������뱽���Ʒ�Ӧ��
D�����Ӻ�Ũ��ˮ��Ӧ�������屽�ӳ�����
��� �⣺A��ľ�����˹�������Һ��ˮ��ҺҲ����ʹľ������ȼ�գ�Ӧ�ü�һ��ľ��������ˮ�ĶԱ�ʵ�����˵�������ƿ���ľ�ķ��������A����
B����̪���뼸�μ��ɣ�����Ҫ����2mL����B����
C�������ӷ������뱽���Ʒ�Ӧ��Ӧ�ȳ�ȥ����C��ȷ��
D��Ӧ��Ũ��ˮ��ϡ��ˮ�۲첻����ɫ��������D����
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰���ʵ����ʡ��к͵ζ������ʵļ���ȣ��������ʵ����ʼ���Ӧԭ��Ϊ���Ĺؼ���ע��ʵ��������Է�������Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2KBr+Cl2=2KCl+Br2 | B�� | CaCO3=CaO+CO2�� | ||
| C�� | SO3+H2O=H2SO4 | D�� | MgCl2+2NaOH=Mg��OH��2��+NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
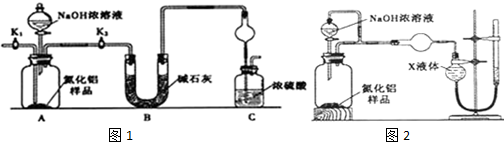
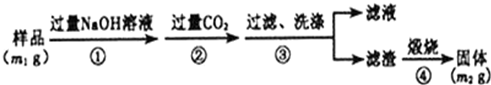
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���д���Fe2+����Һ��Na+��SO42+��NH4+��Fe��CN��63- | |
| B�� | ʹ���ȱ�����Һ��NH4+��CH3COOһ��SO42+��Mg2+ | |
| C�� | ij��ɫ��Һ��OHһ��K+��ClOһ��Ba2+ | |
| D�� | ���д���NO3-����Һ��K+��Iһ��NH4+��H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҵ������������ˮ�ࡢƯ�۾���Ҫ��ʯ��ʯΪԭ�� | |
| B�� | �û���̿Ϊ�ǽ���ɫ���ô�������Ư��ֽ����ԭ����ͬ | |
| C�� | ����ӻ�������֬��������ˮ��Ϊ��������͵�С���Ӳ��ܱ����� | |
| D�� | ˾ĸ�춦����Զ���װ塢�л�������ԭ�������ںϽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
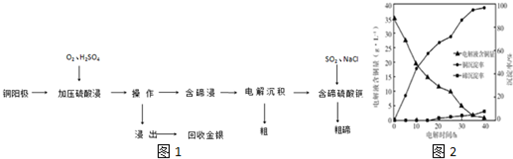
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
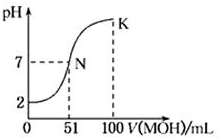 �����£���100mL 0.01mol•L-1HA��Һ����μ���0.02mol•L-1��MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������˵������ȷ���ǣ�������
�����£���100mL 0.01mol•L-1HA��Һ����μ���0.02mol•L-1��MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������˵������ȷ���ǣ�������| A�� | HAΪһԪǿ�ᣬMOHΪһԪ���� | |
| B�� | ����MOH��Һ�����Ϊ50 mLʱ��c��M+����c��A-�� | |
| C�� | N��ˮ�ĵ���̶ȴ���K��ˮ�ĵ���̶� | |
| D�� | K��ʱ��c��MOH��+c��M+��=0.02 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
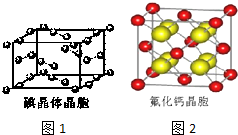 ±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǣ�
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǣ�| �� | �� | �� | �� | |
| ��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 |
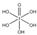 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣮ��Ƚ϶�������ǿ����H5IO6��HIO4�������������������=����
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣮ��Ƚ϶�������ǿ����H5IO6��HIO4�������������������=�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com