
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��NaAlO2��Һ�еμӱ���NaHCO3��Һ���а�ɫ�������� | ��֤���߶�������ˮ�ⷴӦ������ٽ� |
| B | ������Һ�м��뼸��ϡ��ˮû�а�ɫ�������� | ˵����������û�з�����Ӧ |
| C | �����°�����ȼ��������Ҫ�ڷŵ�ʱ����������Ӧ | �ǽ����ԣ�P��N |
| D | ����ͬ�������ͬpH������һԪ���зֱ��������п�ۣ������������������ | ���ԱȽ�����һԪ����������ǿ�� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ� | �ζ����� | ������Һ���/ml | ��NaOH��Һ���������ml�� | |
| �ζ�ǰ/ml | �ζ���/ml | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.30 | 22.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
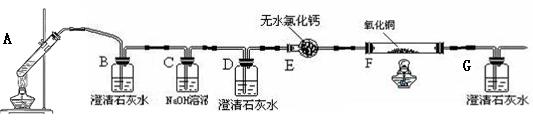 ��1��װ��C�������� _______��װ��E�������� ��
��1��װ��C�������� _______��װ��E�������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
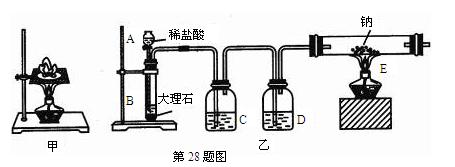
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


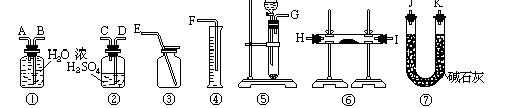
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
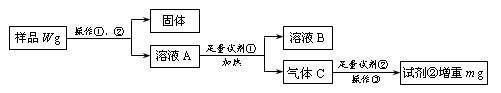
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��ʦ�ڿ�������ʾ������ʵ��װ�ã�����̨�ȸ���������ȥδ������
��ʦ�ڿ�������ʾ������ʵ��װ�ã�����̨�ȸ���������ȥδ������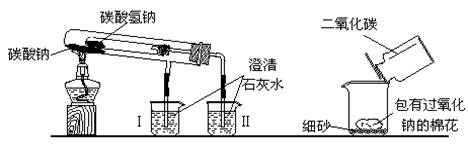
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ�в�һ������SO42�� |
| B | �ýྻ��Ptպȡij��Һ������ɫ��Ӧ������ʻ�ɫ | ����Һ��һ������Na������k�� |
| C | ��CH3CH2Br��NaOH��Һ��ϼ��ȣ��ٵμ�AgNO3��Һ��δ����dz��ɫ���� | CH3CH2Brδ����ˮ�� |
| D | ����ĥ����AlƬͶ��һ��Ũ�ȵ�CuCl2��Һ���������ݲ��й������ɣ����ˣ�������м�������İ�ˮ�����岿���ܽ� | Al��CuCl2��Һ��Ӧ��������H2��Cu(OH)2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com