��2012?���ģ�⣩ij�о�С����̽��SO
2�Ļ�ѧ���ʣ����������ʵ�鷽����
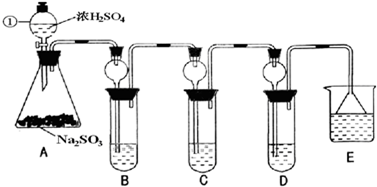
��1��ָ�������ٵ�����
��Һ©��
��Һ©��
��
��2�����Aװ�õ������Եķ�����
�رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A����������
�رշ�Һ©��������������ĩ�˲���B�Թ�ˮ�У�������ס��ƿ�����ڵ��ܿ�������ð�����ɿ��ֺ�������һ��ˮ���������װ��A����������
��
��3��װ��B����SO
2�������ԣ���B����ʢ�Լ�����Ϊ
����ˮ��Һ�������ơ����⻯����Һ���ɣ�
����ˮ��Һ�������ơ����⻯����Һ���ɣ�
��
��4��װ��C��ʢװ��ˮ���Լ���SO
2��
��ԭ
��ԭ
�ԣ���C�з�Ӧ�����ӷ���ʽΪ
SO2+Br2+2H2O=SO42-+4H++2Br-
SO2+Br2+2H2O=SO42-+4H++2Br-
��
��5��װ��D��ʢװ����Ư��Ũ��Һͨ��SO
2һ�΅����D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
�ټ���һ���ð�ɫ����ΪCaSO
3��������ð�ɫ����Ϊ
CaSO4
CaSO4
��
���������ð�ɫ����Ϊ�����������ʵĻ���
�ڻ��ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5mol��L
-1 HCl��0.5mol��L
-1 H
2SO
4��0.5mol��L
-1BaCl
2��1mol��L
-1 NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������ã�
��ش�ϴ�ӳ����ķ�����
�ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ���
�ز�������©���м�����ˮ����û��������ˮ�������ظ�2��3�����ϲ���
��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������
��������������0.5mol��L-1HCl��
��������������0.5mol��L-1HCl��
���Լ��������ϴ����ܵĵ������������ܵ���һ�˲���ʢ��
Ʒ����Һ
Ʒ����Һ
���Լ������Թ��У�
������
��������ȫ�ܽ⣬�����ݲ���������ʹƷ����Һ��ɫ
��������ȫ�ܽ⣬�����ݲ���������ʹƷ����Һ��ɫ
���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��
Ca��ClO��2+H2O+SO2=CaSO4+2HCl
Ca��ClO��2+H2O+SO2=CaSO4+2HCl
��
��6��װ��E��ʢ�ŵ��Լ���
NaOH��Һ
NaOH��Һ
��������
����SO2����ֹ��ɿ�����Ⱦ
����SO2����ֹ��ɿ�����Ⱦ
��





 ��2012?���ģ�⣩������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���ģ�⣩������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������