
��֪п��ϡ���ᷴӦΪ���ȷ�Ӧ��ijѧ��Ϊ��̽���䷴Ӧ�����е����ʱ仯������ˮ�������ռ���Ӧ�ų���������ʵ���¼���£�
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL�� | 30 | 120 | 280 | 350 | 370 |
��1����2��3min �÷�Ӧ�Ƿ��ȷ�Ӧ����ʱ�¶ȸߣ�H+Ũ�ȴ�
��4��5min ��ʱH+Ũ��С
��2��A��C�� B�� Zn �û���Cu���γ�Cu��Znԭ��أ�ʹ��Ӧ����
��������������¶�Խ�ߣ���Ӧ����Խ�죻����Ũ��ԽС����Ӧ����Խ�����������Ӵ��������Ӧ���ʼӿ죬������Һ�м���������NaCl��Һ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С���ݴ˿��Խ��
��1���ٸ��ݱ������ݿ�֪��0��1��l��2��2��3��3��4��4��5min��ʱ�����������������ֱ�Ϊ30��90��160��70��20�����Կ�����Ӧ����������2��3min����������п��ϡ���ᷴӦΪ���ȷ�Ӧ���淴Ӧ�Ľ��У��¶�Խ��Խ�ߣ���Ӧ���ʼӿ졣
�ڷ�Ӧ������С��ʱ���Ϊ4��5min�������������ŷ�Ӧ�Ľ��У�������Ũ��Խ��ԽС����Ӧ����Խ��Խ����
��2��ѡ��A����������Һ�м�������������ˮ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С����Ӧ���ʼ�������A��ȷ��ѡ��B�м����Ȼ�ͭ��Һ��п�û���ͭ���γ�Cu��Znԭ��أ�ʹ��Ӧ���ʸ��죬B����ȷ��ѡ��C��������������Һ�м���������NaCl��Һ���൱�ڼ�ˮ������ϡ�ͣ�����������Ũ�ȼ�С����Ӧ���ʼ�������C��ȷ��
���㣺����Ӱ�컯ѧ��Ӧ�������ص��й�ʵ��̽��
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������ͣ�����������ѧ���淶�Ͻ���ʵ���������������������ѧ����ѧ�����������ѧ����Ӧ������������������Ҫ����ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�״�����Ϊ2l���͵�����ȼ�ϣ���ҵ��ͨ�����з�Ӧ��͢���CH4��H2OΪԭ�����Ʊ��״���
CH4(g)+H2O(g) CO(g)+3H2(g)������ CO(g)+2H2(g)
CO(g)+3H2(g)������ CO(g)+2H2(g) CH3OH(g) ������
CH3OH(g) ������
��1����1.0 mol CH4��2.0 mol H2O(g)ͨ���ݻ�Ϊ100L��Ӧ�ң���һ�������·�����ӦI��CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��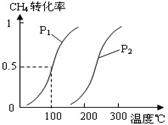
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ ��
��ͼ�е�P1 P2���<������>����=������100��ʱƽ�ⳣ����ֵΪ ��
��2����ѹǿΪ0.1 MPa������, ��a mol CO�� 3a mol H2�Ļ�������ڴ��������£��Է���Ӧ�����ɼ״���
�۸÷�Ӧ�ġ�H 0���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� ��
| A�������¶� | B����CH3OH(g)����ϵ�з��� |
| C������He��ʹ��ϵ��ѹǿ���� | D���ٳ���1mol CO��3mol H2 |
| ʵ���� | T(��) | n(CO)/n(H2) | P��Mpa�� |
| i | 150 | 1/3 | 0.1 |
| ii | | | 5 |
| iii | 350 | | 5 |
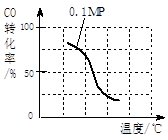
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������(Na2S2O3)�׳ƴ��մ�����ҵ��������Ӱ����Na2S2O3������ˮ����������Һ�����ᷴӦ�е������SO2���ɡ�
��1��Na2S2O3��Һ��ϡ�����Ϸ�Ӧ������̽����������Է�Ӧ���ʵ�Ӱ�죬����йص�ʵ����Ʊ�(��֪����Һ�����Ϊ5 mL)��
| ʵ���� | T/K | c(Na2S2O3)/ mol��L��1 | c(H2SO4)/ mol��L��1 | ʵ��Ŀ�� |
| �� | 298 | 0��1 | 0��1 | ʵ��ٺ͢�̽���¶ȶԸ÷�Ӧ���ʵ�Ӱ�죻 ʵ��ٺ͢�̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�� |
| �� | 308 | | | |
| �� | | 0��2 | |
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����������Թ�A�У�������ˮ�ܽ⡣ | |
| ����2�����Թ�A���� | |
| ����3��ȡ����2�������ϲ���Һ���Թ�B�У� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졣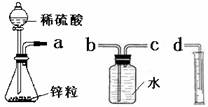
��1��Ϊ�ﵽ��ʵ��Ŀ����װ������˳��Ϊ��a��________��________��________.
��2�����Ӻ�װ�ú����һ��������
��3����ƿ�з�����Ӧ�����ӷ���ʽΪ
��4������װ�õķ�Һ©����װ���Լ��ֱ�Ϊ1mol/L�����4mol/L����,��С��ͬѧҪ�ⶨ����¼���������±���
| ������Լ� | H2���������ͬ�����£� | ��Ӧʱ�� | ��Ӧ���� |
| 1mol/L������ | 10mL | t1 | v1 |
| 4mol/L���� | 10mL | t2 | v2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼΪ��ȡ����������ʵ��װ��ͼ���ش��������⣺ 
��1����ʾʵ��ԭ��
���������Ҵ��ڴ������ڵ������¼��ȿ��Է�����Ӧ��������������������ͬλ��ʾ�ٷ�д���������Ҵ�����������Ӧ�Ļ�ѧ����ʽ______________________��
���ܷ�����ͬλ��ʾ�ٷ���ʾ������Ӧԭ���� _______��ѡ��ܡ����ܡ�����ԭ����___________________��
��2����Ӧ�¶�ȷ����
�ϳ����������ķ�ӦΪ���ȷ�Ӧ��ʵ���������Ӧ�¶�Ӧ������85������Ϊ�ˡ��ش�
ʵ���¶Ȳ��˵���85�����ҵ�ԭ����__________________________________________��
ʵ���¶Ȳ��˸���85�����ҵ�ԭ����__________________________________________��
��3��ʵ��װ�õıȽϣ�
������ͼװ���Ʊ���������������װ����̲�װ����Ƚ�ͻ�����ŵ���__________________________��
��4�������ȵı�ʾ��
Ϊ���õزⶨ�����ȣ���Ԥ����Na2CO3��Һ�еμ�1��____��Һ��������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£���0.1mol/L��̼������Һ�У���������Ũ�ȵĹ�ϵʽ��ȷ����
A��2c(H2CO3)+c(HCO )+c(H+) = c(OH��) )+c(H+) = c(OH��) |
B��c(Na+) = c(HCO )+c(H2CO3)+ 2c(CO )+c(H2CO3)+ 2c(CO ) ) |
C��c(Na+)��c(H+)��c(OH��)��c(CO ) ) |
D��c(Na+)+c(H+) = c(HCO )+c(OH��)+ 2c(CO )+c(OH��)+ 2c(CO ) ) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�Գ�����0.1 mol/L�Ĵ�����Һ������˵����ȷ����
| A����ˮ��������ĵ�c(H+)=1.0��10-13 mol/L |
| B��c(CH3COOH)��c(H+)��c(CH3COO-)��c(OH-) |
| C����ͬŨ�ȵ�����ֱ��ˮϡ��10����pH(����)��pH(����) |
| D�����Ũ�ȵ����NaOH��Һ��Ӧ�����Һ�У�c(CH3COOH)+c(CH3COO-)="0.1" mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и�����Ũ�ȵĴ�С�Ƚϣ���ȷ���ǣ� ��
| A��ͬŨ��������Һ�У��� (NH4)2SO4 �� NH4HCO3 �� NH4Cl�� NH3��H2O�� c(NH4��)�ɴ�С��˳���ǣ��� > �� > �� > �� |
| B������ʱ���������������Ͱ�ˮ��Ϻ�pH = 7����c (NH4+) > c (Cl��) |
| C��0.2 mol��L-1 Na2CO3��Һ�У�c (OH��) =" c" (HCO3��) + c (H+) + c (H2CO3) |
| D��0.01 mol��L-1 ��NH4Cl��Һ��0.05mol��L��1 ��NaOH��Һ�������ϣ� c (Cl��)> c (NH4+)> c (Na+)>c (OH��)>c (H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����Һ�������25�棬�й�������ȷ����
| A��ij���ʵ���ҺpH <7���������һ�������ǿ�������� |
| B���ù㷺pH��ֽ���0.10 mol��L��1 NH4Cl��Һ��pH��5. 2 |
| C��pH=2��CH3COOH��c(H+)��pH=1��CH3COOH��c(H+)��2�� |
| D��AgCl����ͬ���ʵ���Ũ�ȵ�CaCl2��HCl��Һ�е�Ksp��ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com