
����ǰ��һ��ʳ�������㸡�����Ϻ�����°��������������ȴ������㸡�顱�Ļ�ѧ�ɷ�ʵΪ��̼���ƣ�ʹ�ò������������ˣ���̼���Ʊ��������������岻����Σ������̼���ƣ��׳ƹ���˫��ˮ����ѧʽΪ2Na2CO3��3H2O2����һ�����Σ��ǰ�ɫ����״��ĩ�����Էֽ�Ϊ̼���ƺ������⡣ij̽��С���Ʊ���̼���Ʋ��ⶨ��Ʒ��H2O2�ĺ��������Ʊ����̺�װ��ʾ��ͼ���£�
��֪��50 ��Cʱ 2Na2CO3��3H2O2 (s) ��ʼ�ֽ�
����Ӧ 2Na2CO3 (aq) + 3H2O2 (aq) 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0
����Ӧ 2H2O2 = 2H2O + O2��
�ζ���Ӧ 6KMnO4 + 5(2Na2CO3��3H2O2) +19H2SO4 = 3K2SO4 + 6MnSO4+10Na2SO4 + 10CO2 �� + 15O2�� + 34H2O
����������Ϣ�ش��������⣺
��1���Ʋ�ͼ��֧�ܵ����ÿ����� ��
��2������ٵĹؼ��ǿ����¶ȣ����װ��ͼ�������ʩ�� ��
�� ��
��3������ҺX�м�������NaCl�������ˮ�Ҵ�����������̼���ƣ�ԭ���� ��
��4���������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ���� ��
��5���ⶨ��Ʒ��H2O2�����������ķ����ǣ�ȷ��ȡ0.2000g��̼������Ʒ��250 mL ��ƿ�У���50 mL ����ˮ�ܽ⣬�ټ�50 mL 2.000 mol��L��1 H2SO4 (H2SO4����)����0.002000mol��L��1 KMnO4����Һ�ζ����յ�ʱ����30.00 mL��
�ٵζ�ǰ���ζ�������KMnO4����Һ��ϴ2��3�Σ���ϴ�IJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4�� ��
��������Ʒ��H2O2�����������������ʽΪ ��ֻ�г���ʽ�������κ����㣡H2O2��ʽ��Ϊ34.00����
��17�֣�
��1��ƽ��ѹǿ��������Һ˳�����£�2�֣���ƽ��ѹǿ����ѹ����2�֣�����������������֣�
��2����ˮԡ �������� ��ͨ����Һ©��������������Σ��μ�H2O2��Һ����6�֣���2�֡�˵�������������Ŀ����ʹ��Ӧ��������������ɢȥ��
��3�����Ͳ�Ʒ�����̼���ƣ����ܽ��ԣ��ȣ���2�֣���˼�������֣�
��4��ϴȥˮ�ݣ����ڸ��[1��]�����ٹ������ܽ����ʧ[1��]����2�֣�����������������֣���
��5������бת���ζ�����ϴ�����ζ����ڱڣ���1�֣�Ȼ���������ϴҺ���¶˷ų� (1��)[��2�֣�����Ҫ�㣬һ����бת�������Ǵ�������ϴҺ���¶˷ų���û�д�Ҫ�㲻���֡�]
��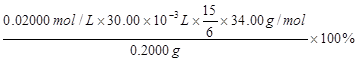 ��3�֣�
��3�֣�
[���չ㶫�߿��������������ʽ��ȫ��ȷ��û����λ��100%���۷֡��������������0�֣�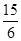 ��2.5���治�۷֣�����������������������2��]
��2.5���治�۷֣�����������������������2��]
���������������1����ͼ���ɺ�ѹ��Һ©���ƶϣ�֧�ܵ�������ƽ��ѹǿ��������Һ˳�����£���2�������⣬����Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��ߵ���H2O2�ֽ⣬��������¶ȣ�����H2O2�ķֽ⣬ͼ������ˮԡ���������������ܷ�ֹ��ӦҺ�¶ȹ��ߣ������¶ȸߵͿ��Ƶ���˫��ˮ���ٶȣ�Ҳ�ܿ��Ʒ�ӦҺ���¶ȣ���3������������Һ�ǹ�̼���Ƶı�����Һ����������NaCl����ˮ�Ҵ������ܽ������ܽ�ȣ�������Һ��������̼���ƹ��壻��4����ˮ�Ҵ�ϴ�ӵ�Ŀ���dz�ȥ�����л��е�ˮ�֣������ڹ���ĸ��ͬʱ���ܼ��ٹ�̼���Ƶ��ܽ⣬������ʧ����5������ϴ��ʽ�ζ��ܵIJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4����Һ����бת���ζ�����ϴ�����ζ����ڱڣ�Ȼ���������ϴҺ���¶˷ų�������n=c?V����ζ�ʱ������0.002000��30.00��10��3mol KMnO4���ζ���ӦΪ6KMnO4+5(2Na2CO3��3H2O2)+19H2SO4=3K2SO4+6MnSO4+10Na2SO4+10CO2��+15O2��+ 34H2O�����ݸ����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ�������������Ĺ�̼���ƣ�2Na2CO3��3H2O2��Ϊ0.002000��30.00��10��3��5/6mol������2Na2CO3��3H2O2=2Na2CO3+3H2O2�����ݸ����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ�����Ʒ��H2O2�����ʵ���Ϊ0.002000��30.00��10��3��5/6��3mol������m=n?M������Ʒ��H2O2������Ϊ0.002000��30.00��10��3��5/6��3��34.00g��������Ʒ����Ϊ0.2000g������Ʒ��H2O2�����������������ʽΪ0.002000��30.00��10��3��5/6��3��34.00/0.2000��100%��
���㣺���������Ʊ��Ĺ������̼���Ʒ���Ȳⶨʵ�飬�漰��������;�������¶ȵķ���������ԭ�ζ��ܵ�ʹ�÷�������Ʒ�й����������������ı���ʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�á�
��1��ʵ������װ��A�Ʊ�SO2��ijͬѧ��ʵ��ʱ���ִ�A�ķ�Һ©��������©����Һ��δ���£�����Ϊԭ������ǣ� ��
��2��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ��ѧ����ʽΪ��
MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
����6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
��3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�B�� ��D�� ��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�ΪB�� ��D�� ��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ���û�ѧ����ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������FeS2��ȼ�ղ�����SO2ͨ�����й��չ��̼����Ƶ�H2SO4�������Ƶ�H2��
��1���ù�������ѭ�����õ�����Ϊ ��
��2���ڸù����У�ʹ��Ĥ��Ӧ����ʱ�����HI�ֽ������H2��Ŀ���� ����ƽ���ƶ���ԭ�����ͣ���
��3��ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3��������������ʵ�顣��֪Ũ����ķе�Ϊ338�棬����ʱ�Ƶƻ�����¶�Ϊ400�桫500�档
�ټ�ͬѧ��װ��I����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᣬ��������ɸð�ɫ�����Ŀ���ԭ�� �������ӷ���ʽ��ʾ����
����ͬѧ��Ϊ��ͬѧ��װ�ò����ƣ�����˸Ľ�װ��II����ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ�飩��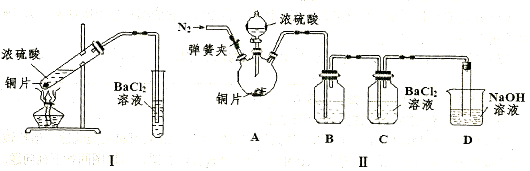
a�����ɼ�ͨ��N2��һ��ʱ���رյ��ɼУ�b���μ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�����a��Ŀ���� ��װ��B�еļ��� ��
�۱�ͬѧȡ��ʵ����C����Һ�������μ�һ����ɫ��Һ����������������İ�ɫ��������ͬѧ�μӵ��Լ������� ������ĸ��ţ���
a��NaOH��Һ b��H2O2��Һ c��������ˮ d������KmnO4��Һ
��װ��D���뵼�����ӵ���Ӳ�ʲ����ܣ���װ��D�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮAlCl3��һ����Ҫ���л��ϳɴ�������������183��ʱ����������ʪ��������������������ij��ѧ��ѧ��ȤС����������ѧ�����������ʵ���Ʊ���ˮAlCl3��ʵ��װ������ͼ��ʾ��
��ش��������⣺
��1���Ʊ�ʵ�鿪ʼʱ���ȼ��װ�õ������ԣ��������IJ��������� ��
a������MnO2��ĩ b����ȼA�оƾ��� c������Ũ���� d����ȼD���ƾ���
��2��д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3��װ��B��C�е��Լ��ֱ��� ��
��4����ͬѧ��ΪF��G������һ������������Ҽ���һ��ҩƷ���ɴﵽ��ͬЧ��������ҩƷ������ ��
��5��E�еõ�������ɫ��ĩ������ľ��������Թ۲쵽��ƿ���а������ɣ��û�ѧ����ʽ��ʾ��ԭ�� ��
��6���Ʊ����������������Ũ���½�����������ȡ��Ӧ��ֹͣ��Ϊ�ⶨ����Һ�������Ũ�ȣ�ijͬѧ��ȡ����Һ10.00mL����ˮϡ�͵�250.00mL��Ȼ�����ȡ��20.00mL����0.1000mol��L-1��NaOH����Һ���еζ����յ�ʱ����NaOH��Һ24.00mL����ò���Һ���������Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��. ��ʵ������������װ�ã����Ʊ�ijЩ���岢��֤�仯ѧ���ʡ�
��������
| ��� | ���� | װ������˳������ĸ�� | �Ʊ���Ӧ�Ļ�ѧ����ʽ |
| ��1�� | ��ϩ | _________________ | _________________ |
| ��2�� | ��Ȳ | A��C��E | _________________ |

 ��_______________��
��_______________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ�Ȼ������л��������ô����������Ϊ��ɫ���壬178��ʱ���������׳��⣬��ˮ��ᷢ�Ȳ�����������ʵ����������װ���Ʊ�������ˮ�Ȼ������䷴Ӧԭ��Ϊ�� 2Al+ 6HCl(g) �� 2A1Cl3 + 3H2��
���������գ�
��1��д����ƿ�У�B����������Ӧ�Ļ�ѧ����ʽ��_____________
��2��C��ʢ�е��Լ�Ϊ_____������ʵ��ʱӦ�ȵ�ȼ_____(ѡ�B����D�������ƾ��ơ�
��3���ô̵ֶ�������D��E��Ŀ����_______ (ѡ�����)��
a�������� b�������� c��ƽ����ѹ d����������
Eƿ��������_______��
��4��F��ʢ�м�ʯ�ң���Ŀ����_______(ѡ�����)��
a������HCl b������Cl2 c������CO2 d������H2O
��5����D�й����Ϊ�����Ȼ�����AlCl3��6H2O)Ҳ�ܽ�����ˮ�Ȼ������Ʊ�����ʱͨ��HCl�����Ŀ����_______����ʵ���������Ʋ��������յõ��������Ǽ�ʽ�Ȼ���[��ѧʽΪAl2(OH)nCl(6-n)]����������ԭ�����Ȼ�����40%���������n��ֵΪ_______��
��6�����˽��齫����װ��A��B�е��Լ���ΪŨ����Ͷ������̣�����װ�ú��Լ������䣬 Ҳ���Ʊ���ˮAlCl3����ʵ֤���������Ƚ�Σ�գ����������_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и���ʵ�����������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ij������Һ����AgNO3��Һ | �а�ɫ���� | �����β�һ����NaCl |
| B | Ũ������NaCl�����ϼ��� | ��������� | ��������Ա�HClǿ |
| C | ij��ɫ����ͨ����ˮ�� | ��ˮ��ɫ | ������һ����C2H4 |
| D | ��Ũ�Ⱦ�Ϊ0.1mol��L��1��NaCl��NaI�����Һ�еμ�������Pb(NO3)2��Һ | ���ֻ�ɫ���� ��PbI2�� | KSP��PbI2����KSP��PbCl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ʵ�顱���м��ס����㡢��Լ����ɫ���ŵ㣬�۲������ĸ������ʵ�顱���г�װ��δ���������ж�����˵����ȷ���� ����������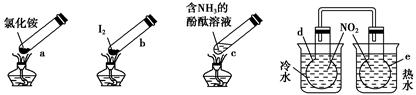
| A������ʱ��a�ϲ��ۼ��˹���NH4Cl��˵��NH4Cl�����ȶ��ԱȽϺ� |
| B������ʱ������b��I2��Ϊ��ɫ���������ϲ��־ۼ�Ϊ�Ϻ�ɫ�Ĺ��� |
| C������ʱ��c����Һ��ɫ�����ȴ���ֱ�dz |
| D��e��������ɫ��dz��d��������ɫ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com