
��10�֣�ijѧ���������ϵ�֪�������������������ȡ�������±���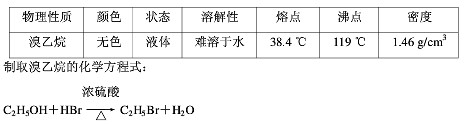
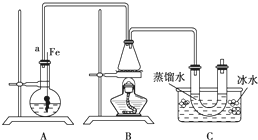
���뵽��ѧ�屽����ȡ����������ȡ�屽���������װ��I����Ҫʵ�鲽�����£�
�ټ�������Ժ�����ƿ�м���һ�����ı���Һ�塣
������ƿ�м����Ҵ���Ũ����Ļ��Һ��ǡ���ڽ������ܿڡ�
�۽�Aװ���еĴ���˿���²�����Һ�С�
�ܵ�ȼBװ���оƾ��ƣ���С������ƿ����10min.����д���пհף�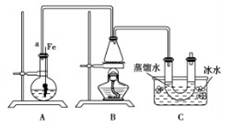
��1��A�з�����Ӧ�ķ���ʽ
��2��װ�ó�����a��������
��3��Cװ���е�U�ι���������ˮ��ס�ܵ�������
��4����Ӧ��Ϻ�U�ι��ڵ������� �����������ʱ���õ�����Ҫ������������ ��ֻ��һ�֣�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧ���������ϵ�֪�������������������ȡ�������±���
ijѧ���������ϵ�֪�������������������ȡ�������±���| �������� | ��ɫ | ״̬ | �ܽ��� | �۵� | �е� | �ܶ� |
| ������ | ��ɫ | Һ�� | ������ˮ | 38.4�� | 119�� | 1.46g/cm3 |
| ||
| �� |
 +Br2
+Br2| Fe |
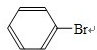 +HBr
+HBr +Br2
+Br2| Fe |
 +HBr
+HBr�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ��ͬʵ����ѧ�߶����£���ĩ��ѧ�Ծ��������棩 ���ͣ������
| �������� | ��ɫ | ״̬ | �ܽ��� | �۵� | �е� | �ܶ� |
| ������ | ��ɫ | Һ�� | ������ˮ | 38.4�� | 119�� | 1.46g/cm3 |
 C2H5Br+H2O
C2H5Br+H2O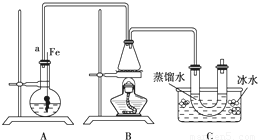
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱����ʮ���и߶����ϣ����л�ѧ�Ծ������ƣ��������棩 ���ͣ������
| �������� | ��ɫ | ״̬ | �ܽ��� | �۵� | �е� | �ܶ� |
| ������ | ��ɫ | Һ�� | ������ˮ | 38.4�� | 119�� | 1.46g/cm3 |
 C2H5Br+H2O
C2H5Br+H2O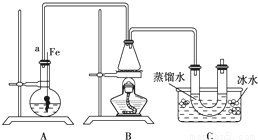
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com