

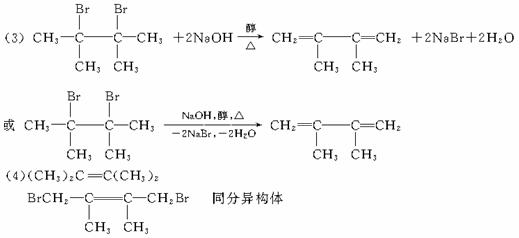
�÷�ӦҲ�ɱ�ʾΪ��
�����ǰ˸��л��������ת����ϵ��

������������⣺
��1������ϵͳ��������������A��������__________________��
��2��������ͼ�У�����________________��Ӧ������________________��Ӧ�����Ӧ���
��3��������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��________________��
��4��C2�Ľṹ��ʽ��________________��
F1�Ľṹ��ʽ��________________��
F1��F2��Ϊ________________��
��5�������˸��������У����ڶ�ϩ������_____________����ϩ����ͨʽ��______________��
��1��2��3-��������
��2��ȡ�� �ӳ�
��3��

��4����CH3��2C=C��CH3��2 ![]() ͬ���칹��
ͬ���칹��
��5��E CnH2n-2
�����������ǰ���ʱȽϼ��ڣ�1���ʿ����л�����������ڣ�2���ʿ����л���Ӧ�Ļ������ͣ��ڣ�3������4�������������йط�Ӧ��������ȷ�����ʵĽṹ����B�Ľṹ����Ӧ������֪C1��C2����Ϊ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2������E�ɷ���1��4-�ӳɺ�1��2-�ӳɣ���EΪ��ϩ���������Ƶ�C2Ϊ��CH3��2C=C��CH3��2��DΪ![]() ������������ӭ�ж����ˡ�
������������ӭ�ж����ˡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�÷�ӦʽҲ�ɱ�ʾΪ

�����ǰ˸��л��������ת����ϵ��

������������⣺
��1������ϵͳ��������������A��������___________��
��2��������ͼ�У�����_________��Ӧ������_________��Ӧ�����Ӧ���ͣ�
��3��������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��______________________��
��4��C2�Ľṹ��ʽ��_________��F1�Ľṹ��ʽ��_________��F1��F2��Ϊ_________��
��5�������˸��������У����ڶ�ϩ������_________����ϩ����ͨʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ɹŰ���һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
±�����ڼ��Դ���Һ���ܷ�����ȥ��Ӧ�����磺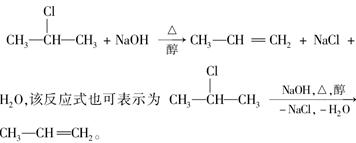
��ͼ�ǰ����л��������ת����ϵ��
��ش��������⣺
(1)������ͼ�У�������ȥ��Ӧ���� ������ţ�.
(2)����________ ______ (�Ӧ����),
(3)������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��
___________________________________________________��
(4)C2�Ľṹ��ʽ��__________________ ��
F1�Ľṹ��ʽ��________________ ��
F1��F2��Ϊ________________��
(5)�������ֻ������У����ڶ�ϩ������________��
��ϩ����ͨʽ��________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ɹŰ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
±�����ڼ��Դ���Һ���ܷ�����ȥ��Ӧ�����磺

��ͼ�ǰ����л��������ת����ϵ��

��ش��������⣺
(1)������ͼ�У�������ȥ��Ӧ���� ������ţ�.
(2)����________ ______ (�Ӧ����),
(3)������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��
___________________________________________________��
(4)C2�Ľṹ��ʽ��__________________ ��
F1�Ľṹ��ʽ��________________ ��
F1��F2��Ϊ________________��
(5)�������ֻ������У����ڶ�ϩ������________��
��ϩ����ͨʽ��________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
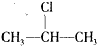 +NaOH
+NaOH  CH3-CH=CH2��+NaCl+ H2O���÷�ӦʽҲ�ɱ�ʾΪ
CH3-CH=CH2��+NaCl+ H2O���÷�ӦʽҲ�ɱ�ʾΪ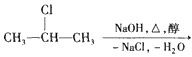 CH3-CH=CH2 ��ͼ�ǰ˸��л��������ת����ϵ
CH3-CH=CH2 ��ͼ�ǰ˸��л��������ת����ϵ 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com