
����Ŀ��ij�����л���A�ķ�����Ϊ88��̼����������Ϊ54.5%�������������Ϊ9.1%������Ϊ�����ɷ�����ͼ��ʾ�仯��
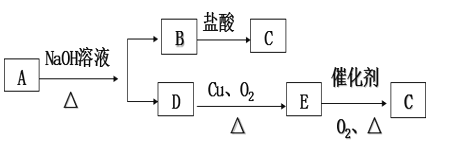
��1��C�й����ŵ�������__________ ��
��2��D��E�ķ�Ӧ������____________��
��3���л���A������������Һ��Ӧ�Ļ�ѧ����ʽΪ___________��
��4����֪ij��X����Է���������AС16��������̼���������֮��Ϊ5:1������˵����ȷ����________��
A.��ͬ������X���ܶȱ�ˮС
B. ��X��������������ԭ��Ӧ
C.��������X��Ϊͬϵ��
D.C��D��E��������������ͭ����
���𰸡��Ȼ� ������Ӧ CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH ACD
��������
�л���A�ķ�����Ϊ88��̼����������Ϊ54.5%�������������Ϊ9.1%������Ϊ��������1molA�к�̼n(C)=88g��54.5![]() ��12g/mol=4mol,����n(H)=88��9.1%��1g/mol=8mol������n(O)= 88g
��12g/mol=4mol,����n(H)=88��9.1%��1g/mol=8mol������n(O)= 88g![]() (1-54.5%
(1-54.5%![]() 9.1%)��16g/mol=2mol������A�ķ���ʽΪ��C4H8O2����A��NaOH��ˮ������AΪ������ͼ��֪BΪ�����ƣ�B�����ᷴӦ�õ�C��DΪ����D�ܷ���������Ӧ����E��E���ܷ���������Ӧ����C����B��DΪ��̼���������ƺʹ�����DΪ�Ҵ���EΪ��ȩ��CΪ�����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��5:1,��X�ķ���ʽΪ��CmHn,����12m
9.1%)��16g/mol=2mol������A�ķ���ʽΪ��C4H8O2����A��NaOH��ˮ������AΪ������ͼ��֪BΪ�����ƣ�B�����ᷴӦ�õ�C��DΪ����D�ܷ���������Ӧ����E��E���ܷ���������Ӧ����C����B��DΪ��̼���������ƺʹ�����DΪ�Ҵ���EΪ��ȩ��CΪ�����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��5:1,��X�ķ���ʽΪ��CmHn,����12m![]() n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������
n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������
��1��������������֪��CΪ���ᣬ����C�Ĺ�����Ϊ�Ȼ���
�����Ϊ���Ȼ���
��2��D��E�ķ�Ӧ���Ҵ��Ĵ�������Ӧ����Ӧ����Ϊ������Ӧ��
�����Ϊ��������Ӧ��
��3���ɷ�����֪�л���AΪ���������������л���A������������Һ��Ӧ�Ļ�ѧ����ʽΪ��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�������CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��4����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��Ϊ5:1,��X�ķ���ʽΪ��CmHn,����12m![]() n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������A.��ͬ������������ܶȱ�ˮС,��A��ȷ��B.����Ϊ�����������ܺ������ӳɣ���B����C. ��������X��Ϊͬϵ���C��ȷ��D. C��D��E�ֱ�Ϊ���ᡢ�Ҵ�����ȩ��������������ͭ������ֱ�Ϊ��������ͭ�ܽ⡢������������ש��ɫ�������ɣ�����ͬ����������������ͭ���飬��D��ȷ��
n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������A.��ͬ������������ܶȱ�ˮС,��A��ȷ��B.����Ϊ�����������ܺ������ӳɣ���B����C. ��������X��Ϊͬϵ���C��ȷ��D. C��D��E�ֱ�Ϊ���ᡢ�Ҵ�����ȩ��������������ͭ������ֱ�Ϊ��������ͭ�ܽ⡢������������ש��ɫ�������ɣ�����ͬ����������������ͭ���飬��D��ȷ��
�������ACD��


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��C2H2��Ϊԭ�Ϻϳ�ʳ������E��������pyrrole����·����ͼ��ʾ�����ַ�Ӧ������������ȥ������D��һ�������¿ɱ�������ͪ��
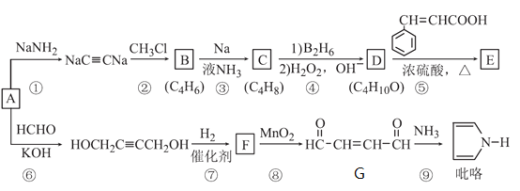
�ش��������⣺
��1��A������������_______________��C��������_______________��
��2���ķ�Ӧ������_______________����ķ�Ӧ������_______________��
��3����Ӧ�ݵĻ�ѧ����ʽΪ____________________________��
��4��������������������ᣨ![]() ����ͬ���칹�干��_________�֣������������칹��:�ٺ��б��� ������������Ȼ�̼��Һ��ɫ ����ˮ�⡣��G��Ϊͬ���칹�壬�Һ˴Ź�������ֻ��һ�����л���Ľṹ��ʽ��________������дһ�֣�
����ͬ���칹�干��_________�֣������������칹��:�ٺ��б��� ������������Ȼ�̼��Һ��ɫ ����ˮ�⡣��G��Ϊͬ���칹�壬�Һ˴Ź�������ֻ��һ�����л���Ľṹ��ʽ��________������дһ�֣�
��6�����������ϳ�·�ߣ����һ����A����ȩΪ��ʼԭ���Ʊ�2,5-����������![]() ���ĺϳ�·��_________________________________��
���ĺϳ�·��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ǧ��PbCrO4����һ��������ˮ�Ļ�ɫ���ϣ�����ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵��������ǣ� ��
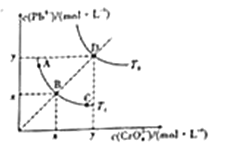
A. ͼ��x��y�ֱ�ΪT1��T2�¶���PbCrO4��ˮ�е��ܽ��
B. ͼ�и����Ӧ��Ksp�Ĺ�ϵΪKsp��A��=Ksp��C��<Ksp��B��<Ksp��D��
C. ��A�����Һ�м�������Na2CrO4���壬��Һ�����A��ABC����B�����ƶ�
D. �¶Ƚ���ʱ��D��ı�����Һ�������D��DB����B�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ԭ�ӵ�������a g ��12Cԭ�ӵ�������b g ����NA��ʾ����ӵ�����������˵������ȷ���ǣ� ��
����Ԫ�ص����ԭ������һ����![]() �� m g����ԭ�ӵ����ʵ���һ����
�� m g����ԭ�ӵ����ʵ���һ����![]() mol�۸���ԭ�ӵ�Ħ��������aNA g/ mol�� a g����ԭ��������������17NA
mol�۸���ԭ�ӵ�Ħ��������aNA g/ mol�� a g����ԭ��������������17NA
A. �٢�B. �٢�C. �ڢ�D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������װ���������ʵ�飨ͼ��a��b��c��d Ϊֹˮ�У���
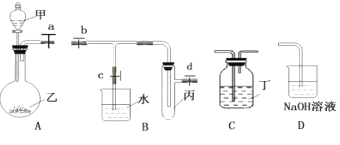
��1�������ҵ�����_________��
��2��װ�� A��B��D ��������֤��SO2��ˮ�е��ܽ�ȣ���ز��������ǣ���ȡ SO2���ռ� SO2�� �ر�ֹˮ�� b��d����ֹˮ�� c��_____________��
��3�����ձ����д���ˮ��������Թ��У�˵�� SO2 ������ˮ�� װ�� A��C ������������֤��C��Si �ķǽ�����ǿ�������Լ�����_____________��C �� �� �� �� ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��15.6gNa2X�к�Na+0.4mol����Na2X��Ħ��������____________��
��2����NAΪ�����ӵ���������ֵ�����a g�����к��еķ�����Ϊb����c g�����ڱ�״���µ����Լ��_________________(�ú�NA��ʽ�ӱ�ʾ����
��3��ij��Һ��Mg2����Al3����Cl����SO42- 4��������ɣ��������Al3����SO42-��Cl�������ʵ���Ũ��֮��Ϊ3��1��13������Һ��Mg2����Al3����SO42-�����ʵ���Ũ��֮��Ϊ___________��
��4������£��ܶ�Ϊ1.25g/L��CO2��CH4��ɵĻ�������У�CO2���������Ϊ______��
��5������״�������ΪaL��HCl��������1000gˮ�У��õ�������ܶ�Ϊbg/cm3��������������ʵ���Ũ��Ϊ______________mol/L��
��6�����и����뽺�������ص���______________________��
��±ˮ�㶹�� ��������ˮ �۾������ ����ˮ���� ��ѪҺ�� ���������γ� ������к� �����ʺ�ͺ�����¥ ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ����Ҫ�Ļ����м��壬������ҵ�о����ȵ㡣һ����ʯ�ͻ����еķ�����������Ҫ�ɷ�ΪNiCO3��SiO2������������Fe2O3��Cr2O3��Ϊԭ���Ʊ��������Ĺ�ҵ������ͼ��

��֪����NiS��Ni(OH)2��Cr(OH)3��������ˮ��Cr(OH)3�������������
��Fe(OH)3������NH4Cl-��ˮ�Ļ��Һ��Ni(OH)2����NH4Cl-��ˮ�Ļ��Һ����[Ni(NH3)6]2+��
��ش��������⣺
��1�������ܡ�ʱӦ�Ƚ������������飬����20%������100���·�Ӧ2h���ò�����Ŀ��Ϊ____��
��2������������Ҫ�ɷ�Ϊ___���ѧʽ�����������ڹ�ҵ�ϵ���;Ϊ___����дһ�֣���
��3����һ�μ�����ʱ�������NaOH��Һ�������������������Ӧ�����ӷ���ʽΪ__��
��4�������⡱��Ŀ��Ϊ___����������ʱͨ��H2S��Ŀ����___��
��5����������ʱ������Ӧ�����ӷ���ʽΪ___��
��6����ϵ�в�����������ָ____�����ˡ�ϴ�ӡ����������NiSO4��7H2O���岻����Ӧ�������е��ᴿ��������Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����ܱ������ڲ���һ��©���ҿɻ����Ļ����������ֳ����������ң������ڳ���һ�������CO��H2O���壬���ҳ���SO2��O2�������壬��SO2��O2�����ʵ���֮��Ϊ2��1�����������ڸ��ɷ������·�Ӧ��
���� CO(��)+H2O(��) ![]() CO2(��)+H2(��)��H<0
CO2(��)+H2(��)��H<0
���� 2SO2(��)+O2(��) ![]() 2SO3(��) ��H<0
2SO3(��) ��H<0
��Ӧ��ʼʱ����ͣ��������˵�3/7������Ӧ�ں����½��У�����ƽ��״̬ʱ���������������м䡣����������ȷ����
![]()
A. �������巴Ӧǰ���ѹǿ֮��Ϊ4:3
B. ��Ӧ��ƽ��ʱ����SO2��ת����Ϊ75��
C. ƽ��ʱ���������г�����ԭ���ʵ���֮����ͬ��CO��H2O���壬��ƽ��ʱ��CO�����ʵ����������� ��SO2�����ʵ����������
D. ��ԭ����������������ʼʱ����ĸ����ʵ�����Ϊԭ������������ƽ��ʱ������������м�λ�ý�ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ�Ӧ�Ⱥ��Ȼ�ѧ��Ӧ����������ȷ����
A. HCl��NaOH��Ӧ���к�����H����57.3 kJ��mol��1����CH3COOH��NaOH��Ӧ���к�����H����57.3 kJ��mol��1
B. CO(g)��ȼ������283.0 kJ��mol��1����Ӧ2CO(g)��O2(g)===2CO2(g)����H����2��283.0 kJ��mol��1
C. ������ȼ����Ϊ285.5 kJ��mol��1������ˮ���Ȼ�ѧ����ʽΪ2H2O(l)![]() 2H2(g)��O2(g)�� ��H����2��285.5 kJ��mol��1
2H2(g)��O2(g)�� ��H����2��285.5 kJ��mol��1
D. 1 mol����ȼ��������̬ˮ�Ͷ�����̼ʱ���ų��������Ǽ����ȼ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com