
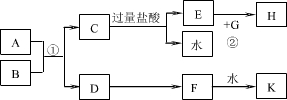
£Ø15·Ö£©ÖŠŃ§»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĒŅŅŃÖŖ½«FµÄ±„ŗĶČÜŅŗµĪČė·ŠĖ®ÖŠ£¬Öó·ŠæɵƵ½ŅŌHĪŖ·ÖÉ¢ÖŹµÄŗģŗÖÉ«½ŗĢ唣
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŗģŗÖÉ«½ŗĢåÖŠHĮ£×ÓµÄÖ±¾¶“óŠ”·¶Ī§ £»
£Ø2£©Š“³öDµÄŅ»ÖÖ¹¤ŅµÓĆĶ¾£ŗ £»
£Ø3£©¢ŁŠ“³öH2O2µÄµē×ÓŹ½£ŗ £»
¢ŚŠ“³öB”śGµÄĄė×Ó·½³ĢŹ½ £»
£Ø4£©ČōA”¢BĒ”ŗĆĶźČ«·“Ó¦£¬½«²śĪļCÓė×ćĮæŃĪĖį·“Ó¦µĆµ½a molĘųĢ壬ĮķČ”µČÖŹĮæBÓė×ćĮæŃĪĖį·“Ó¦µĆµ½b molĘųĢ壬a:b=5:7£¬ŌņAµÄ»ÆѧŹ½ĪŖ £»
£Ø5£©ÓĆŹÆÄ«×÷µē¼«£¬µē½āG¼ÓČė¹żĮæŃĪĖįŗóµÄČÜŅŗ£ØČēÓŅĶ¼£©£¬Į½¼«²śÉśĘų ÅŻ”£³ÖŠųµē½āŅ»¶ĪŹ±¼ä£¬ŌŚX¼«ø½½üµÄČÜŅŗÖŠ»¹æɹŪ²ģµ½µÄĻÖĻó ŹĒ £¬½āŹĶ“ĖĻÖĻóµÄĄė×Ó·½³ĢŹ½ŹĒ ”£
¶ĻæŖµēŌ“£¬½«µē½āŗóµÄČÜŅŗµ¹ČėÉÕ±ÖŠ³ä·Ö½Į°č£¬ĻÖĻóŹĒ £¬ŌŅņŹĒ ”£
£Ø15·Ö£©
£Ø1£©1nm ~ 100nm £Ø1·Ö£©
£Ø2£©µē½āŅ±Į¶ĀĮ”¢ÄĶøßĪĀ²ÄĮĻµČ£ØĘäĖūŗĻĄķ“š°øŅ²æÉ£©£Ø1·Ö£©
£Ø3£© ¢Ł £»£Ø2·Ö£©¢Ś2Al+2OH-+2H2O=2AlO2-+3H2”ü£Ø2·Ö£©
£Ø4£©Fe5O7£»£Ø2·Ö£©
£Ø5£©ĻČ³öĻÖ°×É«»ė×Ē£¬ŗóÓÖ±ä³ĪĒå £Ø2·Ö£© Al3++3OH-=3Al(OH)3”ż£¬Al(OH)3+ OH-= AlO2-+2H2O£Øø÷1·Ö£©³öĻÖ°×É«³Įµķ £Ø1·Ö£© Ńō¼«ĒųµÄAl3+ÓėŅõ¼«ĒųÉś³ÉµÄAlO2-Ē”ŗĆ·“Ӧɜ³ÉAl(OH)3£Ø»ņÕß“š”°øł¾Ż×Ü·“Ó¦£ŗ2AlCl3+ 6H2O 2Al(OH)3+3H2”ü+3Cl2”ü£¬×īŗóĒ”ŗĆÉś³ÉAl(OH)3³Įµķ”£”±ŅąµĆ·Ö£©£Ø2·Ö£©
½āĪö:ĀŌ


 ×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾(Ķ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļ¼°·“Ó¦Ģõ¼žĪ“ĮŠ³ö)”£
 |
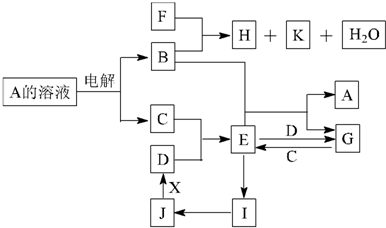
ŅŃÖŖ£ŗA”¢B”¢C”¢D”¢EŹĒµ„ÖŹ£¬×é³ÉĖüĆĒµÄŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪĪŖa”¢b”¢c”¢d”¢e£»ĒŅ3(a + b) = 2(a + c) = 3(d £a)£¬X”¢Y”¢Z”¢M”¢N”¢W”¢H”¢KŹĒ»ÆŗĻĪļ£¬ĘäÖŠXŹĒBŗĶCµÄ»ÆŗĻ²śĪļµÄĖ®ČÜŅŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÓƵē×ÓŹ½±ķŹ¾XÖŠČÜÖŹµÄŠĪ³É¹ż³Ģ£ŗ_______________£¬×é³ÉCµÄŌŖĖŲµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ŹĒ_____ ”£
£Ø2£©Š“³öBŌŚŃõĘųÖŠČ¼ÉÕÉś³ÉµÄ²śĪļÓėH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_______________”£
£Ø3£©×é³Éµ„ÖŹB”¢C”¢DµÄČżÖÖŌŖĖŲ¼ņµ„Ąė×ӵĥė×Ó°ė¾¶Óɓ󵽊”µÄĖ³ŠņŹĒ__ _(ÓĆĄė×Ó·ūŗűķŹ¾)”£
£Ø4£©Š“³öKÓė¹żĮæµÄ°±Ė®·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»Š“³öMŌŚĖ®ČÜŅŗÖŠµēĄėµÄ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£©£ŗ
ŅŃÖŖ£ŗB”¢C”¢DĪŖ³£¼ūµ„ÖŹ£¬ĘäÖŠCĪŖĘųĢ壬B”¢DĪŖ½šŹō£»FĪŖ³£¼ūµÄĒæĖį£»K³£ĪĀĻĀĪŖĘųĢ壬ÄæÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«£»½« E ČÜŅŗµĪČė·ŠĖ®æÉÖʵĆŅ»ÖÖŗģŗÖÉ«½ŗĢ壻 J ĪŖŗģ×ŲÉ«¹ĢĢ唣Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
 |
( l ) B µÄ»ÆѧŹ½ĪŖ £»
Š“³ö B Óė F ·“Ó¦µÄ»Æѧ·½³ĢŹ½
( 2£©ŹµŃéÖŠ±£“ę G ČÜŅŗŹ±ŅŖ¼ÓČė ÄæµÄŹĒ
( 3£©ĪŖŹµĻÖJŅ»DµÄ±ä»Æ£¬ČōXŹĒ·Ē½šŹōµ„ÖŹ£¬ŌņXæÉÄÜŹĒ £ØŠ“»ÆѧŹ½£©; ČōXŹĒ½šŹōµ„ÖŹ£¬ĒėŠ“³ö J Ņ» D ·“Ó¦µÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com