
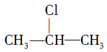 +NaOH$”ś_{”÷}^{“¼}$CH3-CHØTCH2+NaCl+H2O£¬
+NaOH$”ś_{”÷}^{“¼}$CH3-CHØTCH2+NaCl+H2O£¬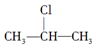 $”ś_{-NaCl£¬-H_{2}O}^{NaOH”¢“¼”¢”÷}$CH3-CHØTCH2ĻĀĆęŹĒ¼øÖÖÓŠ»ś»ÆŗĻĪļµÄ×Ŗ»Æ¹ŲĻµ£ŗ
$”ś_{-NaCl£¬-H_{2}O}^{NaOH”¢“¼”¢”÷}$CH3-CHØTCH2ĻĀĆęŹĒ¼øÖÖÓŠ»ś»ÆŗĻĪļµÄ×Ŗ»Æ¹ŲĻµ£ŗ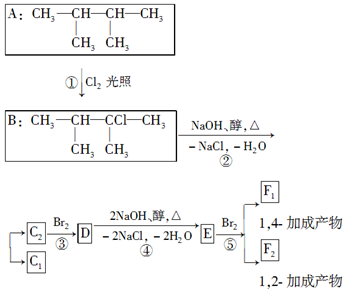
·ÖĪö AŌŚ¹āÕÕĢõ¼žĻĀÓėĀČĘų·¢ÉśČ”“ś·“Ӧɜ³ÉB£¬BŌŚĒāŃõ»ÆÄĘ“¼ČÜŅŗ”¢¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ӧɜ³ÉC1”¢C2£¬C2Óėäå·¢Éś¼Ó³É·“Ӧɜ³ÉD£¬DŌŚĒāŃõ»ÆÄĘ“¼ČÜŅŗ”¢¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ӧɜ³ÉE£¬EÓėäåæÉŅŌ·¢Éś1£¬2-¼Ó³É·“Ӧɜ³ÉF2£¬·¢Éś1£¬4-¼Ó³É·“Ӧɜ³ÉF1£¬ŌņEĪŖCH2=C£ØCH3£©C£ØCH3£©=CH2”¢F2ĪŖCH2=C£ØCH3£©CBr£ØCH3£©CH2Br£¬F1ĪŖBrCH2C£ØCH3£©=C£ØCH3£©CH2Br£®ÄęĶĘæɵĆDĪŖCH3CBr£ØCH3£©CBr£ØCH3£©2£¬C2ĪŖ£ØCH3£©2C=C£ØCH3£©2£¬C1ĪŖ£ØCH3£©2CHC£ØCH3£©=CH2£®
½ā“š ½ā£ŗ£Ø1£©ÓÉ»ÆŗĻĪļAµÄ½į¹¹¼ņŹ½£¬ŌņĆū³ĘŹĒ£ŗ2£¬3-¶ž¼×»ł¶”Ķ飬¹Ź“š°øĪŖ£ŗ2£¬3-¶ž¼×»ł¶”Ķ飻
£Ø2£©ÉĻŹöæņĶ¼ÖŠ£¬·“Ó¦¢ŁŹōÓŚČ”“ś·“Ó¦£¬·“Ó¦¢ŪŹōÓŚ¼Ó³É·“Ó¦£¬¹Ź“š°øĪŖ£ŗČ”“ś£»¼Ó³É£»
£Ø3£©øł¾ŻŅŌÉĻ·ÖĪö£¬DÉś³ÉEµÄ»Æѧ·½³ĢŹ½£ŗCH3CBr£ØCH3£©CBr£ØCH3£©2+2NaOH$”ś_{”÷}^{“¼}$CH2=C£ØCH3£©C£ØCH3£©=CH2+2NaBr+2H2O£¬
¹Ź“š°øĪŖ£ŗCH3CBr£ØCH3£©CBr£ØCH3£©2+2NaOH$”ś_{”÷}^{“¼}$CH2=C£ØCH3£©C£ØCH3£©=CH2+2NaBr+2H2O£»
£Ø4£©øł¾ŻŅŌÉĻ·ÖĪö£¬C2µÄ½į¹¹¼ņŹ½ŹĒ£ŗ£ØCH3£©2C=C£ØCH3£©2£¬F1µÄ½į¹¹¼ņŹ½ŹĒ£ŗBrCH2C£ØCH3£©=C£ØCH3£©CH2Br£¬F2µÄ½į¹¹¼ņŹ½ŹĒ£ŗCH2=C£ØCH3£©CBr£ØCH3£©CH2Br£¬F1ÓėF2»„ĪŖĶ¬·ÖŅģ¹¹Ģå
¹Ź“š°øĪŖ£ŗ£ØCH3£©2C=C£ØCH3£©2£»BrCH2C£ØCH3£©=C£ØCH3£©CH2Br£»Ķ¬·ÖŅģ¹¹Ģ壮
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬ÄѶČÖŠµČ£¬ŹģĮ·ÕĘĪÕ¹ŁÄÜĶŵĊŌÖŹÓė×Ŗ»ÆŹĒ¹Ų¼ü£¬¶ŌѧɜµÄĀß¼ĶĘĄķÓŠŅ»¶ØµÄŅŖĒó£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
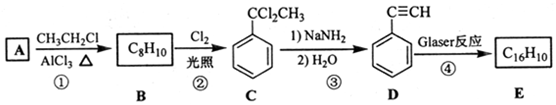
 £¬D µÄ»ÆѧĆū³ĘĪŖ±½ŅŅČ²£®
£¬D µÄ»ÆѧĆū³ĘĪŖ±½ŅŅČ²£® £®ÓĆ1mol EŗĻ³É1£¬4-¶ž±½»ł¶”Ķ飬ĄķĀŪÉĻŠčŅŖĻūŗÄĒāĘų4mol£®
£®ÓĆ1mol EŗĻ³É1£¬4-¶ž±½»ł¶”Ķ飬ĄķĀŪÉĻŠčŅŖĻūŗÄĒāĘų4mol£®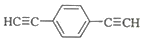 £©Ņ²æÉ·¢ÉśGlaserżĮŖ·“Ӧɜ³É¾ŪŗĻĪļ£¬øĆ¾ŪŗĻ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖn
£©Ņ²æÉ·¢ÉśGlaserżĮŖ·“Ӧɜ³É¾ŪŗĻĪļ£¬øĆ¾ŪŗĻ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖn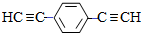 $\stackrel{“߻ƼĮ}{”ś}$
$\stackrel{“߻ƼĮ}{”ś}$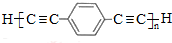 +£Øn-1£©H2£®
+£Øn-1£©H2£®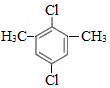 £¬
£¬ £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ļ”ĮņĖįµĪŌŚĶʬÉĻ£ŗCu+2H+ØTCu2++H2”ü | |
| B£® | Ńõ»ÆĆ¾ÓėĻ”ŃĪĖį»ģŗĻ£ŗMgO+2H+ØTMg2++H2O | |
| C£® | Ķʬ²åČėĻõĖįŅųČÜŅŗÖŠ£ŗCu+Ag+ØTCu2++Ag | |
| D£® | Ļ”ŃĪĖįµĪŌŚ“óĄķŹÆÉĻ£ŗCO32-+2H+ØTCO2”ü+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČėŃĪĖį£¬²śÉśÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĪŽÉ«ĘųĢ壬ŌņŹŌŃłÖŠŅ»¶ØÓŠCO32- | |
| B£® | ĻņAgCl³ĮµķÖŠµĪČėĻ”KIČÜŅŗ°×É«³Įµķ±ä»Ę£¬ĖµĆ÷AgI±ČAgCløüÄŃČÜ | |
| C£® | ĻČ¼ÓČėŃĪĖįĪŽ³Įµķ£¬ŌŁ¼ÓČėBaCl2ČÜŅŗ²śÉś°×É«³Įµķ£¬ŌņŹŌŃłÖŠŅ»¶ØÓŠSO42- | |
| D£® | ŃĪČÜŅŗÖŠ¼ÓČėNaOHĪ¢ČČ£¬²śÉśŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ŌņŅ»¶ØŹĒļ§ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĖ®¼ų±š¼×Ėį”¢ŅŅČ©”¢ŅŅĖį | |
| B£® | ³żČ„ŅŅĶéÖŠÉŁĮæµÄŅŅĻ©£ŗ¹āÕÕĢõ¼žĻĀĶØČėĒāĘų | |
| C£® | ³żČ„ŅŅ“¼ÖŠÉŁĮæµÄŅŅĖį£ŗ¼ÓČė×ćĮæµÄÉśŹÆ»Ņ£¬¹żĀĖ | |
| D£® | ÓĆČ¼Éյķ½·Ø¼ų±šŅŅ“¼”¢±½ŗĶĖÄĀČ»ÆĢ¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Č«²æ | B£® | ¢Ū¢Ü¢Ż | C£® | ³ż¢ÜŅŌĶā | D£® | ¢Ś¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ō¶Ńóŗ£ĀÖµÄĶāæĒĮ¬½ÓŠææéæɱ£»¤ĀÖ“¬²»ŹÜøÆŹ“ | |
| B£® | ĢśÖĘĘ÷¼žŌŚ³±ŹŖæÕĘųÖŠÉśŠā | |
| C£® | ¶ĘŠæĢśĘ¬±Č¶ĘĪżĢśĘ¬øüÄĶøÆŹ“ | |
| D£® | ½šŹōĀĮŌŚæÕĘųÖŠ²»Ņ×±»øÆŹ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com