
(17��) �����зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳɰ�����Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����
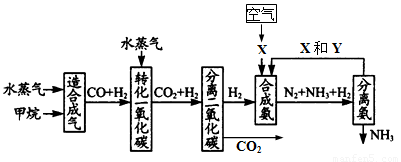
����д���пհף�
(1)��֪2mol������ˮ������t�桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(2)ͼ��XΪ_____��YΪ_____���ѧʽ��������K2CO3��Һ���շ������CO2�������ӷ���ʽΪ_____________________________________
(3)�ںϳɰ���ҵ�У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ��������������û�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�_______________________________��
(4)�����Ƽ�У��ϳɰ�������NH3��CO2ͨ�뱥��ʳ��ˮ���տ��Ƶô����ͼ��ʾ
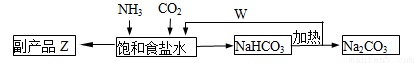
��Ӧ����ʳ��ˮͨ����_______���壨д��ѧʽ����ͬ��������ƷZΪ______��������______��WΪ_______��
�������� Na2CO3 5.3�֣����������ٿ��Ƶø���ƷZ_______�֡�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ij
Щת�����輰������δ�г�����
����д���пհף�
��1����֪0.5 mol�����0.5 molˮ������t �棬p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ______��
��2�����������У���ҵ�Ϸ���H2 ��CO2�����ķ�����___________��
A.�������ͨ������������Һ��������Һ�м�����
B.�������ѹ��ȴ��ʹCO2Һ��
C.������ð�ˮϴ��
D.�������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬
��3��Ϊ�˱�֤����˳���ϳɣ��ڿ�������ϳ���֮ǰ����Կ�������___________,Ŀ����________________________________________���ںϳɰ���ʵ�����������У�����ȡ�����ɵİ��ӻ�������з����������������ķ����� ��
��4��������������Դ����������߾���Ч�棬����Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı��֣������߶κͼ�ͷ����ͼ�е���������������Դ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(17��) �����зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳɰ�����Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����
����д���пհף�
(1)��֪2mol������ˮ������t�桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(2)ͼ��XΪ_____��YΪ_____���ѧʽ��������K2CO3��Һ���շ������CO2�������ӷ���ʽΪ_____________________________________
(3)�ںϳɰ���ҵ�У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ��������������û�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�_______________________________��
(4)�����Ƽ�У��ϳɰ�������NH3��CO2ͨ�뱥��ʳ��ˮ���տ��Ƶô����ͼ��ʾ
��Ӧ����ʳ��ˮͨ����_______���壨д��ѧʽ����ͬ��������ƷZΪ______��������______��WΪ_______��
�������� Na2CO3 5.3�֣����������ٿ��Ƶø���ƷZ_______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ϴ�ѧ������ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(17��) �����зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳɰ�����Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����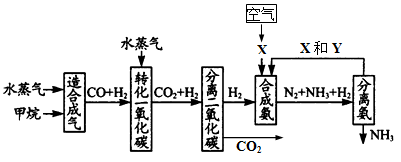
����д���пհף�
(1)��֪2mol������ˮ������t�桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(2)ͼ��XΪ_____��YΪ_____���ѧʽ��������K2CO3��Һ���շ������CO2�������ӷ���ʽΪ_____________________________________
(3)�ںϳɰ���ҵ�У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ��������������û�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�_______________________________��
(4)�����Ƽ�У��ϳɰ�������NH3��CO2ͨ�뱥��ʳ��ˮ���տ��Ƶô����ͼ��ʾ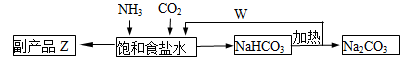
��Ӧ����ʳ��ˮͨ����_______���壨д��ѧʽ����ͬ��������ƷZΪ______��������______��WΪ_______��
�������� Na2CO3 5.3�֣����������ٿ��Ƶø���ƷZ_______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ̨���и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(16��)�ҹ��зḻ�ĺ�ˮ��Դ�����������ú�ˮ��Դ�ǵ�ǰ��ѧ�о���һ����Ҫ������ͼ��ij�������Ժ�ˮ��Դ�ۺ����õ�ʾ��ͼ��
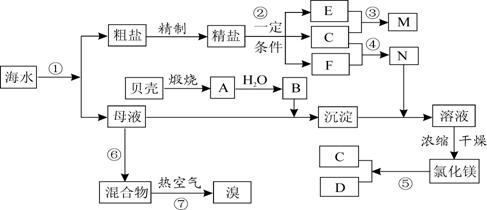
�����������Ϣ�ش��������⣺
I����1��д��N�Ļ�ѧʽ��B�����ƣ�N ��B ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ������������ת�Ƶķ������Ŀ��
��3��д����Ӧ�۵����ӷ���ʽ�� ��
�����к���Ca2����Mg2����SO42-�����ʣ�����ʱ���õ��Լ�Ϊ�������� ���Ȼ�����Һ
������������Һ ��̼������Һ�������Լ����ӵ�˳�����Ϊ ��
A. �ڢۢܢ� B. �ۢܢڢ� C. �ܢۢڢ� D. �ۢڢܢ�
����ȡ���κ�ʣ��ĺ�ˮ��ĸҺ���У���������ȡMg��Br2��
��1����������ȡMg������������ȡMg�����̣�û���漰���ķ�Ӧ������ ��
A���ֽⷴӦ B�����Ϸ�Ӧ C���û���Ӧ D�����ֽⷴӦ
��2����������ȡBr2����Ӧ�����õ���̬��������Ѱ�һ�Դ�����룬���к������� ��
A������ع��� B���ڵ����½�������
C���ӱ��������ռѭ�� D���ӱ�������þ���ʴ�ѭ��
���̢߽��嵥�ʴӻ�����з�������ǻ����嵥�ʾ��� �ԡ�
��3��ĸҺ������ȡMg��Br2�Ⱥ�˳������λ����ʦ�в�ͬ�۵㣺
�ף�ĸҺ����ȡMg������ȡBr2
�ң�ĸҺ����ȡBr2������ȡMg
�����ж��ĸ������ʣ� (��ס����ҡ�)�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com