
����Ŀ��������(NH3BH3)�������ߡ����ȶ��Ժã���һ�־���DZ���Ĺ��崢����ϡ��ش��������⣺
(1)H��B��N�У�ԭ�Ӱ뾶������______�����ݶԽ��߹���B��һЩ��ѧ������Ԫ��______�����ơ�
(2)NH3BH3�����У�N��B��ѧ����Ϊ____��������Ӷ���____�ṩ���������ڴ���������ˮ���ͷ�������3NH3BH3+6H2O=3NH3+![]() +9H2��
+9H2��![]() �Ľṹ��ͼ��ʾ��
�Ľṹ��ͼ��ʾ��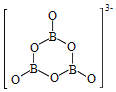 ���ڸ÷�Ӧ�У�Bԭ�ӵ��ӻ����������______��Ϊ______��
���ڸ÷�Ӧ�У�Bԭ�ӵ��ӻ����������______��Ϊ______��
(3)NH3BH3�����У���Nԭ��������H��������(H��+)����Bԭ��������H�ʸ�����(H��-)���縺�Դ�С˳����__________����NH3BH3ԭ��������ȵĵȵ�������_________(д����ʽ)�����۵��NH3BH3____________(����������������)��ԭ������NH3BH3����֮�䣬����____________________��Ҳ����˫�������
(4)�о����֣��������ڵ��¸�ѹ������Ϊ������ϵ�ṹ�����������ֱ�Ϊa pm��b pm��c pm����=��=��=90�����������2��2��2�������ṹ��ͼ��ʾ��
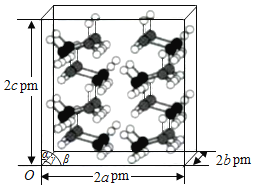
�����龧����ܶ���=___________g��cm3(�г�����ʽ����NAΪ�����ӵ�������ֵ)��
���𰸡�B Si(��) ��λ N sp3 sp2 N��H��B CH3CH3 �� H��+��H���ľ������� ![]()
��������
����Ԫ�������ڱ��е�λ�ñȽϺ��ж�Ԫ�ص�������ʣ���������ԭ�ӵļ۲���Ӷ���ȷ�����ӻ���������ͣ����õ��������ķ���Ѱ�ҵȵ����壻���ݵ縺�ԶԻ��ϼ۵�Ӱ��Ƚϲ�ͬԪ�صĵ縺�ԣ����ݾ��������������������ܶȡ�
(1)������Ԫ���У�Hԭ�ӵİ뾶����С�ģ�ͬһ���ڴ����ң�ԭ�Ӱ뾶���μ�С�����ԣ�H��B��N��ԭ�Ӱ뾶�����B��B��Si��Ԫ�����ڱ��д��ڶԽ��ŵ�λ�ã����ݶԽ��߹���B��һЩ��ѧ������SiԪ�����ơ�
(2)Bԭ���������3�����ӣ�����3��Hԭ���γɹ��ۼ�����۲���Ӷ�ֻ��3�ԣ�����һ���չ������NH3�У�Nԭ����һ�Թ¶Ե��ӣ�����NH3BH3�����У�N��B��Ϊ��λ��������Ӷ���Nԭ���ṩ��NH3BH3�����У�Bԭ�ӵļ۲���Ӷ���Ϊ4�������ӻ���ʽΪsp3��NH3BH3�ڴ�����������ˮ������������B3O63-����ͼ����Ϣ��֪��B3O63-��ÿ��Bԭ��ֻ�γ�3�����������е�Bԭ�ӵ��ӻ���ʽΪsp2����ˣ�Bԭ�ӵ��ӻ����������sp3��Ϊsp2��
(3) NH3BH3�����У���Nԭ��������H�������ԣ�˵��N�ĵ縺�Դ���H����Bԭ��������H�ʸ����ԣ�˵��H�ĵ縺�Դ���B�����3��Ԫ�ص縺���ɴ�С��˳��ΪN��H��B��NH3BH3��������8��ԭ�ӣ���۵�������Ϊ14��N��B�ļ۵�������ƽ��ֵΪ4�����ݵ���������ԭ�����ҵ���ȵ�����ΪCH3CH3������NH3BH3�������ڼ��Է��ӣ���CH3CH3���ڷǼ��Է��ӣ�������Է��������ӽ������Ǽ��Է��ӵķ��Ӽ��������ϴ�CH3CH3�۵��NH3BH3�͡�NH3BH3���Ӽ������˫����������������γ�ԭ����˵������Ӽ����H��+��H��-�ľ���������
(4)�ڰ������222�ij������ṹ�У�����16����������ӣ������ij��������߷ֱ�Ϊ2apm��2bpm��2cpm��������ƽ����Ϊ8�ݿ��Եõ�8��С�����壬��ƽ��ÿ��С��������ռ��2����������ӣ�С������ij��������߷ֱ�Ϊapm��bpm��cpm����С�����������Ϊ![]() ��С����������Ϊ
��С����������Ϊ![]() ����ˣ������龧����ܶ�Ϊ
����ˣ������龧����ܶ�Ϊ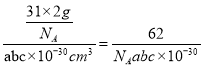 gcm-3��
gcm-3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й��Ȼ�ѧ����ʽ��д���Ӧ��������ȷ����(����)
A. ϡ������0.1 mol��L NaOH��Һ��Ӧ��H+(aq)+OH��(aq)= H2O(l)��H = +57.3 kJ��mol-1
B. ��101KPa��������ȼ������H =��285.5 kJ��mol-1����ˮ�ֽ���Ȼ�ѧ����ʽ��2H2O(l)=2H2(g)+O2(g) ��H = +285.5 kJ��mol-1
C. ��֪2C(s)+O2(g)=2CO(g) ��H=��221 kJ��mol-1�� ���֪C��ȼ���ȴ���110.5 kJ��mol-1
D. 2N2O5(g)![]() 4NO2(g)+O2(g) ��H>0 ��ӦΪ������Ӧ���κ��¶������Է�����
4NO2(g)+O2(g) ��H>0 ��ӦΪ������Ӧ���κ��¶������Է�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���淴Ӧ��0��2���ӽ��й����У� �ڲ�ͬ��Ӧʱ ������ʵ����ı仯�������ͼ��ʾ����÷�Ӧ�ĵķ�Ӧ����______����������_______����ѧ����ʽΪ_____________________________����Ӧ��ʼ��2����ʱ���ܷ���C��ʾ��Ӧ���ʣ����ܣ��䷴Ӧ����Ϊ______________�������ܣ�����ԭ��Ϊ________________________________________________________��2���Ӻ�A��B��C�����ʵ���������ʱ��ı仯���仯��˵������������£���Ӧ�Ѵﵽ��___________״̬��
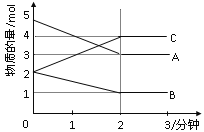
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.1mol�л���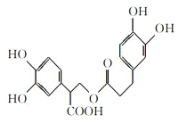 ��һ���������ܺ�7molNaOH��Ӧ
��һ���������ܺ�7molNaOH��Ӧ
B.![]() ����̼ԭ�ӿ��ܶ���ͬһƽ����
����̼ԭ�ӿ��ܶ���ͬһƽ����
C.����ʽΪC4H7ClO2������NaHCO3����CO2���л���Ľṹ��3��
D.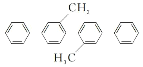 ��һ�ȴ�����9��(�����������칹)
��һ�ȴ�����9��(�����������칹)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̼������ϳ���ϩ���ۺ�����CO2���ȵ��о����ش��������⣺
(1)CO2������������ϩ��ˮ�ķ�Ӧ�У���������ʵ���֮��n(C2H4)��n(H2O)=__________������Ӧ�ﵽƽ��ʱ��������ѹǿ����n(C2H4)___________(�������������С������������)��
(2)���ۼ��������ԭ�ϳ�ʼ���n(CO2)��n(H2)=1��3������ϵѹǿΪ0.1MPa����Ӧ�ﵽƽ��ʱ��������ֵ����ʵ�������x���¶�T�ı仯��ͼ��ʾ��
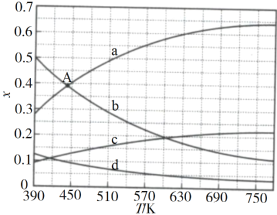
ͼ�У���ʾC2H4��CO2�仯�����߷ֱ���______��______��CO2������ϳ�C2H4��Ӧ����H______0(��������������С����)��
(3)����ͼ�е�A(440K��0.39)��������¶�ʱ��Ӧ��ƽ�ⳣ��Kp=_________(MPa)3(�г�����ʽ���Է�ѹ��ʾ����ѹ=��ѹ�����ʵ�������)��
(4)������̼������ϳ���ϩ��Ӧ�������渱��Ӧ������C3H6��C3H8��C4H8�ȵ�̼����һ���¶Ⱥ�ѹǿ�����£�Ϊ����߷�Ӧ���ʺ���ϩѡ���ԣ�Ӧ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȿ��γɶ��ֺ������Σ��㷺Ӧ����ɱ������������������ʵ������������ͼװ��(����װ��ʡ��)�Ʊ�KClO3��NaClO��̽����������ԭ���ʡ�
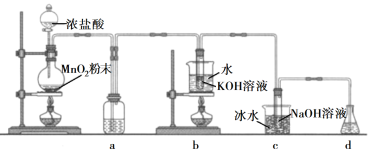
�ش��������⣺
(1)ʢ��MnO2��ĩ������������________��a�е��Լ�Ϊ________��
(2)b�в��õļ��ȷ�ʽ��_________��c�л�ѧ��Ӧ�����ӷ���ʽ��________________�����ñ�ˮԡ��ȴ��Ŀ����____________��
(3)d��������________����ѡ���Լ�________(����)��
A��Na2S B��NaCl C��Ca(OH)2 D��H2SO4
(4)��Ӧ������ȡ��b���Թܣ�����ȴ�ᾧ��________��__________������õ�KClO3���塣
(5)ȡ����KClO3��NaClO��Һ�ֱ�����1�ź�2���Թ��У��μ�����KI��Һ��1���Թ���Һ��ɫ���䡣2���Թ���Һ��Ϊ��ɫ������CCl4�����ú�CCl4����____ɫ����֪��������KClO3����������____NaClO(��������������С��")��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭת¯�̻���Ҫ����Zn�ۻ���������Fe(+2��)��Pb��Cu��As��Ԫ�أݵ������κ����������Ϊ�����Ρ��Ʊ���Ҫ����ԭ�ϻ�������п�Ĺ���������ͼ��ʾ����ش��������⣺
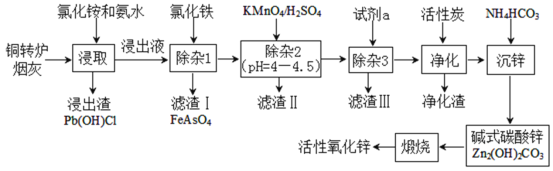
��֪������̿������Ҫ�dz�ȥ�л����ʡ�
(1)д���Ȼ�淋ĵ���ʽ___������������������___(����������������ѧ��)�仯��
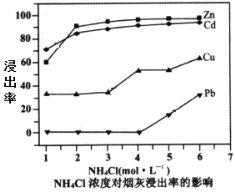
(2)�ڷ�Ӧ�¶�Ϊ50������Ӧʱ��Ϊ1hʱ���ⶨ��Ԫ�صĽ��������Ȼ����ҺŨ�ȵĹ�ϵ��ͼ�����Ȼ�����˵�Ũ��Ϊ___mol��L-1��������Һ��пԪ����[Zn(NH3)4]2+��ʽ���ڣ����ȡʱZnO������Ӧ�����ӷ���ʽΪ___��
(3)�μ�KMnO4��Һ��MnO2���ɣ�Ŀ���dz�___Ԫ�أ�����3���û����ӹ��̣����Լ�a��___��������������Ҫ�ɷ�Ϊ___(�ѧʽ)��
(4)д������п��ʱ������Ӧ�����ӷ���ʽ___���˹����п���ѭ�����õĸ���Ʒ��___��
(5)ȡmg��������п��Ʒ��ɴ���Һ������ָʾ��3��4�Σ��ټ����������Ǽ��İ�����amolL-1EDTA��Һ���еζ������ı�ҺVmL����֪����1.0mLEDTA��Һ[c(EDTA)=1.000mo1L-1]�൱���Կ˱�ʾ������п����Ϊ0.08139������Ʒ������п����������Ϊ___(�ô���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д���¶Է�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��1���Ҵ�����ϩ��__����Ӧ���ͣ�__��
��2���Ҵ���Ũ�����ᷴӦ��__����Ӧ����__��
��3��������Ũ��ˮ��Ӧ��__����Ӧ����__��
��4����ȩ������������ͭ����Һ��Ӧ��__����Ӧ����__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��Ľṹ��ʽ�ǣ�CH2![]() CHCOOH����д�������������ʷ�Ӧ�ķ���ʽ��
CHCOOH����д�������������ʷ�Ӧ�ķ���ʽ��
��1������������Һ________________________________________��
��2����ˮ________________________________________��
��3���Ҵ�________________________________________��
��4������________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com