
ÓŠŅ»ŗ¬NaCl”¢Na2CO3”¤10H2OŗĶNaHCO3µÄ»ģŗĻĪļ£¬Ä³Ķ¬Ń§Éč¼ĘČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬Ķعż²āĮæ·“Ó¦²śÉśµÄCO2ŗĶH2OµÄÖŹĮ棬Ą“Č·¶ØøĆ»ģŗĻĪļÖŠø÷×é·ÖµÄÖŹĮæ·ÖŹż”£
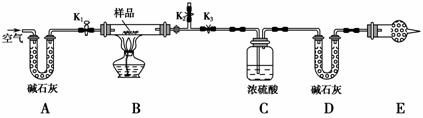
(1)ŹµŃé²½Öč£ŗ
¢Ł°“Ķ¼(¼Š³ÖŅĒĘ÷Ī“»³ö)×é×°ŗĆŹµŃé×°ÖĆŗó£¬Ź×ĻČ½ųŠŠµÄ²Ł×÷ŹĒ____________________”£
¢Ś³ĘȔѳʷ£¬²¢½«Ęä·ÅČėÓ²ÖŹ²£Į§¹ÜÖŠ£»³ĘĮæ×°ÅØĮņĖįµÄĻ“ĘųĘæCµÄÖŹĮæŗĶ×°¼īŹÆ»ŅµÄUŠĪ¹ÜDµÄÖŹĮ攣
¢Ū“ņæŖ»īČūK1”¢K2£¬¹Ų±ÕK3£¬»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬ĘäÄæµÄŹĒ______________________”£
¢Ü¹Ų±Õ»īČūK1”¢K2£¬“ņæŖK3£¬µćČ¼¾Ę¾«µĘ¼ÓČČÖĮ²»ŌŁ²śÉśĘųĢ唣װÖĆBÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________
____________________________________________________Ӣ
___________________________________________________ӣ
¢Ż“ņæŖ»īČūK1£¬»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬Č»ŗó²šĻĀ×°ÖĆ£¬ŌŁ“Ī³ĘĮæĻ“ĘųĘæCµÄÖŹĮæŗĶUŠĪ¹ÜDµÄÖŹĮ攣
(2)¹ŲÓŚøĆŹµŃé·½°ø£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£
¢ŁČō¼ÓČČ·“Ó¦ŗó²»¹ÄČėæÕĘų£¬¶Ō²ā¶Ø½į¹ūµÄÓ°ĻģŹĒ______________________ _______________________________________________________”£
¢ŚE“¦øÉŌļ¹ÜÖŠŹ¢·ÅµÄŅ©Ę·ŹĒ________£¬Ęä×÷ÓĆŹĒ___________________£¬Čē¹ūŹµŃé֊ƻӊøĆ×°ÖĆ£¬Ōņ»įµ¼ÖĀ²āĮæ½į¹ūNaHCO3µÄÖŹĮæ·ÖŹż________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
¢ŪČōѳʷ֏ĮæĪŖw g£¬·“Ó¦ŗóC”¢D×°ÖĆŌö¼ÓµÄÖŹĮæ·Ö±šĪŖm1 g”¢m2 g£¬Ōņ»ģŗĻĪļÖŠNa2CO3”¤10H2OµÄÖŹĮæ·ÖŹżĪŖ________(ÓĆŗ¬w”¢m1”¢m2µÄ“śŹżŹ½±ķŹ¾)”£
½āĪö””±¾Ģāæ¼²éæ¼Éś¶ŌŌŖĖŲ»ÆŗĻĪļµÄČĻŹ¶ŗĶĄķ½āŅŌ¼°¶ŌŹµŃ鏿¾ŻµÄ·ÖĪö“¦ĄķÄÜĮ¦”£
(1)¢Ł×é×°ŗĆŹµŃé×°ÖĆŗóŹ×ĻČÓ¦¼ģ²é×°ÖĆĘųĆÜŠŌ”£¢ŪÓÉӌװÖĆÖŠ“ęŌŚCO2ŗĶĖ®ÕōĘų£¬Ó¦ĻČ¹ÄČėæÕĘų³żČ„×°ÖĆÖŠµÄCO2ŗĶĖ®ÕōĘų”£¢ÜÓÉĪļÖŹµÄŠŌÖŹæÉÖŖøĆ×°ÖĆŌŚ¼ÓČČŹ±·¢ÉśµÄ·“Ó¦ĪŖ2NaHCO3 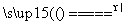 Na2CO3£«H2O”ü£«CO2”ü”¢Na2CO3”¤10H2O
Na2CO3£«H2O”ü£«CO2”ü”¢Na2CO3”¤10H2O 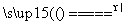 Na2CO3£«10H2O”ü”£
Na2CO3£«10H2O”ü”£
(2)¢Ł¼ÓČČŗóÓŠ²æ·ÖCO2ŗĶĖ®ÕōĘų»į²ŠĮōŌŚ×°ÖĆÖŠ£¬±ŲŠė¹ÄČėæÕĘųŹ¹ĘäÅųöĶźČ«±»ĪüŹÕ£¬Čō²»¹ÄČėæÕĘų£¬Ōņ²āµĆµÄNaHCO3ŗĶNa2CO3”¤10H2OµÄÖŹĮæ·ÖŹżĘ«Š”£¬NaClµÄÖŹĮæ·ÖŹżĘ«“󔣢Ś×°ÖĆEŹĒ·ĄÖ¹æÕĘųÖŠµÄCO2ŗĶĖ®ÕōĘų½ųČė×°ÖĆD£¬¹ŹøÉŌļ¹ÜÖŠŹ¢·ÅµÄŅ©Ę·ŹĒ¼īŹÆ»Ņ£¬Čē¹ūƻӊøĆ×°ÖĆ£¬»įŹ¹²āµĆµÄNaHCO3µÄÖŹĮæ·ÖŹżĘ«“󔣢ŪÓÉĢāÄæŠÅĻ¢ÖŖ·“Ó¦·Å³öµÄCO2µÄÖŹĮæĪŖm2 g£¬øł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½2NaHCO3 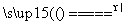 Na2CO3£«H2O”ü£«CO2”ü£¬æɼĘĖć³öøĆ·“Ó¦ÖŠ²śÉśµÄĖ®µÄÖŹĮæĪŖ
Na2CO3£«H2O”ü£«CO2”ü£¬æɼĘĖć³öøĆ·“Ó¦ÖŠ²śÉśµÄĖ®µÄÖŹĮæĪŖ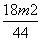 g£¬“Ó¶ų¼ĘĖć³öNa2CO3”¤10H2O·Ö½ā²śÉśĖ®µÄÖŹĮæĪŖ
g£¬“Ó¶ų¼ĘĖć³öNa2CO3”¤10H2O·Ö½ā²śÉśĖ®µÄÖŹĮæĪŖ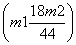 g£¬ŌŁøł¾ŻNa2CO3”¤10H2O
g£¬ŌŁøł¾ŻNa2CO3”¤10H2O 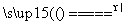 Na2CO3£«10H2O”ü£¬¼ĘĖć³öNa2CO3”¤10H2OµÄÖŹĮæĪŖ
Na2CO3£«10H2O”ü£¬¼ĘĖć³öNa2CO3”¤10H2OµÄÖŹĮæĪŖ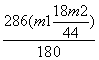 £¬×īŗó¼ĘĖć³ö»ģŗĻĪļÖŠNa2CO3”¤10H2OµÄÖŹĮæ·ÖŹżĪŖ
£¬×īŗó¼ĘĖć³ö»ģŗĻĪļÖŠNa2CO3”¤10H2OµÄÖŹĮæ·ÖŹżĪŖ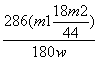 ”Į100%”£
”Į100%”£
“š°ø””(1)¢Ł¼ģ²é×°ÖĆĘųĆÜŠŌ””¢Ū³żČ„×°ÖĆÖŠµÄĖ®ÕōĘųŗĶ¶žŃõ»ÆĢ¼
¢Ü2NaHCO3 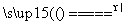 Na2CO3£«H2O”ü£«CO2”ü
Na2CO3£«H2O”ü£«CO2”ü
Na2CO3”¤10H2O 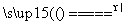 Na2CO3£«10H2O”ü
Na2CO3£«10H2O”ü
(2)¢ŁNa2CO3”¤10H2OŗĶNaHCO3µÄÖŹĮæ·ÖŹż²ā¶Ø½į¹ūĘ«Š”£¬NaClµÄÖŹĮæ·ÖŹż²ā¶Ø½į¹ūĘ«“ó
¢Ś¼īŹÆ»Ņ””·ĄÖ¹æÕĘųÖŠµÄCO2ŗĶĖ®ÕōĘų½ųČėDÖŠÓ°Ļģ²ā¶Ø½į¹ū””Ę«“ó””
¢Ū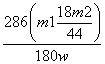 ”Į100%
”Į100%


 ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½²»ÕżČ·µÄŹĒ (””””)”£
A£®ŹÆÓ¢ÓėÉÕ¼ī·“Ó¦£ŗSiO2£«2OH£===SiO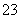 £«H2O
£«H2O
B£®¹čÓėÉÕ¼ī·“Ó¦£ŗSi£«2OH£===SiO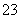 £«H2”ü
£«H2”ü
C£®¹čĖįÄĘČÜŅŗÖŠĶØČėÉŁĮæCO2£ŗSiO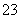 £«CO2£«H2O===CO
£«CO2£«H2O===CO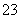 £«H2SiO3”ż
£«H2SiO3”ż
D£®ĶłĖ®²£Į§ÖŠ¼ÓČėŃĪĖį£ŗ2H£«£«SiO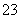 ===H2SiO3”ż
===H2SiO3”ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķعż¶ŌŹµŃéĻÖĻóµÄ¹Ū²ģ”¢·ÖĪöĶĘĄķµĆ³öÕżČ·µÄ½įĀŪŹĒ»ÆѧѧĻ°µÄ·½·ØÖ®Ņ»”£¶ŌĻĀĮŠŹµŃéŹĀŹµµÄ½āŹĶÕżČ·µÄŹĒ (””””)”£
| ²Ł×÷”¢ĻÖĻó | ½āŹĶ | |
| A | ĻņKIµķ·ŪČÜŅŗÖŠ¼ÓČėFeCl3ČÜŅŗ£¬ČÜŅŗ±äĄ¶ | Fe3£«ÄÜÓėµķ·Ū·¢ÉśĻŌÉ«·“Ó¦ |
| B | °ŃÉśĢś·ÅÖĆÓŚ³±ŹŖµÄæÕĘųÖŠ£¬Ģś±ķĆęÓŠŅ»²ćŗģ×ŲÉ«µÄ°ßµć | ĢśŌŚ³±ŹŖµÄæÕĘųÖŠŅ×Éś³ÉFe(OH)3 |
| C | ĻņĻ”ĻõĖįÖŠ¼ÓČėÉŁĮæĢś·Ū£¬ÓŠĘųÅŻ²śÉś | ĖµĆ÷FeÖĆ»»³öĻõĖįÖŠµÄĒā£¬Éś³ÉĮĖĒāĘų |
| D | ŠĀÖĘFe(OH)2Ā¶ÖĆÓŚæÕĘųÖŠŅ»¶ĪŹ±¼ä£¬°×É«ĪļÖŹ±ä³ÉĮĖŗģŗÖÉ« | ĖµĆ÷Fe(OH)2Ņ×±»O2Ńõ»Æ³ÉFe(OH)3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėæĘѧ”¢¼¼Źõ”¢Éē»į”¢»·¾³ĆÜĒŠĻą¹Ų”£ĻĀĮŠÓŠ¹ŲĖµ·ØÖŠÕżČ·µÄŹĒ (””””)”£
A£®Š”ĖÕ“ņæÉÓĆÓŚÉś²ś²£Į§£¬Ņ²æÉÓĆĄ“³żČ„ĪļĘ·±ķĆęµÄÓĶĪŪ
B£®¹żŃõ»ÆÄĘæÉÓĆÓŚŹ³Ę·”¢ÓšĆ«ŗĶÖÆĪļµČµÄĘÆ°×
C£®Ņ½ÓĆ¾Ę¾«”¢“ĪĀČĖįÄʵČĻū¶¾Ņŗ¾łæÉŅŌ½«²”¶¾Ńõ»Æ¶ų“ļµ½Ļū¶¾µÄÄæµÄ
D£®Ź¹ÓĆŗ¬ÓŠĀČ»ÆÄʵÄČŚŃ©¼Į»į¼ÓæģĒÅĮŗµÄøÆŹ“
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
120 mLŗ¬ÓŠ0.20 molĢ¼ĖįÄʵÄČÜŅŗŗĶ200 mL ŃĪĖį£¬²»¹Ü½«Ē°ÕßµĪ¼ÓČėŗóÕߣ¬»¹ŹĒ½«ŗóÕßµĪ¼ÓČėĒ°Õߣ¬¶¼ÓŠĘųĢå²śÉś£¬µ«×īÖÕÉś³ÉµÄĘųĢåĢå»ż²»Ķ¬£¬ŌņŃĪĖįµÄÅضČŗĻĄķµÄŹĒ (””””)”£
A£®2.0 mol”¤L£1”” B£®1.5 mol”¤L£1
C£®0.18 mol”¤L£1”” D£®0.24 mol”¤L£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
O3¾ßÓŠĒæŃõ»ÆŠŌ£¬½«O3ĶØČėKIČÜŅŗÖŠ·¢Éś·“Ó¦£ŗO3£«I££«H£«ØD”śI2£«O2£«H2O(Ī“ÅäĘ½)£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®ÅäĘ½ŗóµÄĄė×Ó·½³ĢŹ½ĪŖ2O3£«2I££«4H£«===I2£«2O2£«2H2O
B£®ĆæÉś³É1 mol I2×ŖŅʵē×Ó2 mol
C£®O2ŹĒ»¹Ō²śĪļÖ®Ņ»
D£®øĆ·“Ó¦ÄÜĖµĆ÷O2µÄŃõ»ÆŠŌ“óÓŚI2µÄ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻ“Ó4J29ŗĻ½š(ĢśīÜÄųŗĻ½š)·ĻĮĻÖŠĢįČ”īÜŗĶÄų£¬Ņ»°ćĻČÓĆĮņĖįČܽāŗĻ½šŹ¹Ö®³ÉĪŖFe2£«”¢Co2£«”¢Ni2£«£¬ŌŁ°ŃFe2£«Ńõ»ÆĪŖFe3£«£¬“Ó¶ųŹ¹Fe3£«×Ŗ»ÆĪŖijÖÖ³ĮµķĪö³ö£¬“ļµ½ÓėNi2£«”¢Co2£«·ÖĄėµÄÄæµÄ”£Éś²śÉĻŅŖŹ¹Fe2£«Ńõ»ÆĪŖFe3£«£¬¶ų²»Ź¹Co2£«”¢Ni2£«±»Ńõ»ÆµÄŹŌ¼ĮŹĒNaClO»ņNaClO3(¾łŗ¬ÉŁĮæH2SO4)ČÜŅŗ£¬·“Ó¦µÄ²æ·Ö»Æѧ·½³ĢŹ½ČēĻĀ(AĪŖ»¹Ō¼Į)£ŗ
NaClO£«A£«BØD”śNaCl£«C£«H2O
NaClO3£«A£«BØD”śNaCl£«C£«H2O
(1)ĒėĶź³ÉŅŌÉĻ»Æѧ·½³ĢŹ½£ŗ________________________________________£¬________________________________________________”£
Źµ¼ŹÉś²śÖŠ²ÉÓĆNaClO3Ą“Ńõ»ÆFe2£«±Č½ĻŗĻĖć£¬ĘäĄķÓÉŹĒ_________________ _____________________________________________________________________________________________________________________________”£
(2)ÅäĘ½ĻĀĮŠĄė×Ó·½³ĢŹ½£¬²¢»Ų“šĪŹĢā”£
 Fe(OH)3£«
Fe(OH)3£« ClO££«
ClO££« OH£===
OH£=== FeO
FeO £«
£« Cl££«
Cl££« H2O
H2O
(3)ŅŃÖŖÓŠ3.21 g Fe(OH)3²Ī¼Ó·“Ó¦£¬¹²×ŖŅĘĮĖ5.418”Į1022øöµē×Ó£¬Ōņn£½________”£
(4)øł¾ŻÉĻŹö(2)(3)ĢāĶĘ²āFeO ÄÜÓėĻĀĮŠÄÄŠ©ĪļÖŹ·“Ó¦________(Ö»ĢīŠņŗÅ)”£
ÄÜÓėĻĀĮŠÄÄŠ©ĪļÖŹ·“Ó¦________(Ö»ĢīŠņŗÅ)”£
A£®Cl2 B£®SO2
C£®H2S D£®O2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖijӊ»śĪļAµÄŗģĶā¹āĘ×ŗĶŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ(””””)
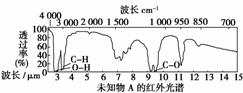
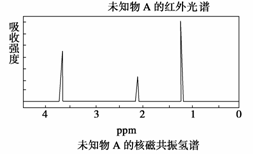 ””””””
””””””
A£®ÓÉŗģĶā¹āĘ×æÉÖŖ£¬øĆÓŠ»śĪļÖŠÖĮÉŁÓŠČżÖÖ²»Ķ¬µÄ»Æѧ¼ü
B£®ÓÉŗĖ“Ź²ÕńĒāĘ×æÉÖŖ£¬øĆÓŠ»śĪļ·Ö×ÓÖŠÓŠČżÖÖ²»Ķ¬»Æѧ»·¾³µÄĒāŌ×Ó
C£®ÓÉĘäÖŹĘ×Ķ¼æÉŅŌµĆÖŖA·Ö×ÓµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ46
D£®×ŪŗĻ·ÖĪöAµÄ»ÆѧŹ½ĪŖC2H6O£¬ŌņĘä½į¹¹¼ņŹ½ĪŖCH3”ŖO”ŖCH3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ×éŗĻÖŠŌŚČĪŗĪĪĀ¶ČĻĀ·“Ó¦¾łÄÜ×Ō·¢½ųŠŠµÄŹĒ £Ø £©
A£®”÷H>0£¬”÷S>0 B£®”÷H<0£¬”÷S<0
C£®”÷ H>0£¬”÷ S<0 D£®”÷ H<0£¬”÷ S>0
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com