
����Ŀ��ij��ʵ������480mL��0.5mol/L��ϡH2SO4��Һ��ijͬѧ��98%��ŨH2SO4(��=1.84g/cm3)�������ƣ���ش��������⣺
(1)ʵ����Ҫ�IJ������������ձ�����Ͳ��������������____________��
(2)���㣺98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��Ϊ_____________�����Ʊ���ʵ����Ҫ��ϡ����������Ͳ��ȡ����98%��ŨH2SO4_______ mL��
(3)���ƹ��̣�������Ͳ��ȡ�����Ũ���ᡣ
�ڽ�Ũ���Ỻ��ע��ʢ����������ˮ���ձ��У��ӱ߽��衣
���ò������������ձ��е���Һת�Ƶ��Ѿ���©�ĺ��ʹ�������ƿ�С�
��ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲע������ƿ�У�����ҡ������ƿ��ʹ��Һ��Ͼ��ȡ�
��������ƿ�м�������ˮ���ھ���̶�1��2cmʱ�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
�Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�߽����ƺõ���Һת�����Լ�ƿ�д��á�
����������Һ�IJ������ȱʧ��ȱ�ٵIJ�����__________________��Ӧ���ڲ���_____֮ǰ����(���������)��
(4)�ں�������д���и����������������ҺŨ�ȵ�Ӱ��(ѡ����ƫ��������ƫ����������Ӱ����).
����ȡŨ����������Ͳ������ˮ_________��
�ڶ���ʱ������Һ��_________��
������Ͳ��ȡŨ����ʱ����Һ��___________��
���𰸡���ͷ�ιܡ�500mL����ƿ 18.4mol/L 13.6 ���ձ��е���Һ��ȴ������ �� ƫ�� ƫ�� ƫ��
��������
(1)����һ�����ʵ���Ũ�ȵ���Һ��һ��Ҫʹ��һ����������ƿ����ʵ����û��480mL������ƿ������ѡ��500mL������ƿ������ʱ��ʹ�ý�ͷ�ιܡ�
(2)����98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��ʱ��ʹ�ù�ʽ![]() ���м��㣻���Ʊ���ʵ����Ҫ��ϡ���ᣬ������ϡ�Ͷ��ɣ���������98%��ŨH2SO4�������
���м��㣻���Ʊ���ʵ����Ҫ��ϡ���ᣬ������ϡ�Ͷ��ɣ���������98%��ŨH2SO4�������
(3)���ƹ���ʱ���������Ʋ��裺���㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȣ��������������ҳ���ȱʧ�IJ��輰����λ�á�
(4)�Ӳ���������ȡ��Ũ��������������Һ�����Ӱ�죬��������������ҺŨ�ȵ�Ӱ�졣
(1)ʵ����Ҫ�IJ������������ձ�����Ͳ��������������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
(2)98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��Ϊ![]() mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6���ʴ�Ϊ��18.4mol/L��13.6��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6���ʴ�Ϊ��18.4mol/L��13.6��
(3)��������һ�����ʵ���Ũ�ȵ���Һ���裺���㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȡ�ȱ�ٵIJ����ǽ��ձ��е���Һ��ȴ�����£��ò���Ӧ���ڲ����֮ǰ���У��ʴ�Ϊ�����ձ��е���Һ��ȴ�����£��ۣ�
(4)����ȡŨ����������Ͳ������ˮ���൱��ϡ����Ũ���ᣬ������ȡ��Ũ��������������ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ڶ���ʱ������Һ�棬������Һ�����ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
������Ͳ��ȡŨ����ʱ����Һ�棬������ȡ��Ũ��������ƫ����������ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ͣ�ƫ�ߣ�ƫ�ߡ�


 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����װ�ķ��ݻ��ͷų��ж��ļ�ȩ���塣��-Ferrozine������ȩ��HCHO����ԭ�����£���ԭ�����������������ȩ�ķ�Ӧ��������˵����ȷ����

A.����������HCHO��Fe3+�����ʵ���֮��Ϊ4:1
B.30gHCHO������ʱ�������ϵ�·��ͨ��2mol����
C.��������ĵ缫��ӦʽΪAg2O+2H++2e-=2Ag+H2O
D.����������ͬ����ȩŨ��Խ��������ɫ�������Һ�������ԽС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ɴ�����Ⱦ����Ҫ���ʡ��о���������ķ�Ӧ������������������Ⱦ����Ҫ���塣
(1)��֪��N2(g)��O2(g)=2NO(g)����H1����180.5 kJ��mol��1
C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
2C(s)��O2(g)=2CO(g)����H3����221 kJ��mol��1
��Ӧ2NO(g)��2CO(g)N2(g)��2CO2(g)����H��________ kJ��mol��1��
(2)��������ȥ��NO����һ�������£���NH3����NO��Ⱦ���䷴Ӧԭ��Ϊ4NH3��6NO![]() 5N2��6H2O����ͬ�¶������£�n(NH3)��n(NO)�����ʵ���֮�ȷֱ�Ϊ4��1��3��1��1��3ʱ���õ�NO�ѳ���������ͼ��ʾ��
5N2��6H2O����ͬ�¶������£�n(NH3)��n(NO)�����ʵ���֮�ȷֱ�Ϊ4��1��3��1��1��3ʱ���õ�NO�ѳ���������ͼ��ʾ��
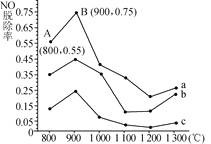
��n(NH3)��n(NO)�����ʵ���֮��Ϊ1��3ʱ����Ӧ��������________(����a����b������c��)��
����ͼ��֪�������Ժ��ֱ�����Ӧ�����¶ȳ���900��ʱNO�ѳ��ʶ�����Ȼ�½���ԭ�������____________��
(3)NO������Ӧ��2NO(g)��O2(g)2NO2(g)���������У��䷴Ӧ���������仯ʾ��ͼ��ͼ��

��. 2NO(g)��N2O2(g)����H1��
��. N2O2(g)��O2(g)��2NO2(g)����H2
�ٻ�ѧ��Ӧ���������ʽ����ķ�Ӧ������������Ϸ�Ӧ����NO������Ӧ���ʵIJ�����________(����������������)��
���ں��ݵ��ܱ������г���һ������NO��O2���壬���������������䣬���Ʒ�Ӧ�¶ȷֱ�ΪT3��T4(T4>T3)�����c(NO)��t(ʱ��)�ı仯������ͼ��ת����ͬ����NO�����¶�_____(����T3������T4��)�����ĵ�ʱ��ϳ����Խ�Ϸ�Ӧ��������ͼ������ԭ��______��

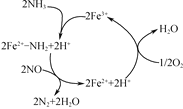
(4) NH3����ԭNO����Ҫ�����������������䷴Ӧ������������ϵ��ͼ��

�о���������Fe2O3Ϊ���Ĵ����Ͽ��ܷ����ķ�Ӧ������ͼ��д���������̵��ܷ�Ӧ����ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�������5 minʱ�����ͭ�缫����������2.16 g���Իش�

��1����Դ��Y����____(����������������)����C�����缫�����ĵ缫��Ӧʽ��___
��2��ͨ��5 minʱ��B�й��ռ���224 mL(��״��)���壬B�����������ĵ缫��Ӧʽ___����Һ���Ϊ200 mL(���ǰ����Һ������仯���Բ���)����ͨ��ǰc(CuSO4)��______��
��3����A��KCl��Һ�����Ҳ��200 mL��������Һ������Cl��������5 min��,��������������Ϊ����״����___����ʱ��Һc(OH-)��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������и�����
(1)��������9�����ʣ��������������������������������壻���������Ȼ��������������أ����Ȼ�����Һ�����ƾ�(C2H5OH)���������ơ�
����Ӧ��ѧʽ��д���пհף������������������____�����ڵ���ʵ���______�����ڷǵ���ʵ�_______��(����Ӧ��ѧʽ���)
���й�����������˵����ȷ����____(����ĸ���)��
a�������ȶ����ܷ⾲�û�������� b�����ܲ��������ЧӦ����������
c����ɢ�����Ӷ�����ͨ����ֽ d���������ᶼ���Ȳ������������ܽ�
(2)��״���£�2.24 L Cl2������Ϊ__________����__________����ԭ�ӡ�
(3)10.8g R2O5����ԭ�ӵ���ĿΪ3.01��1023�� ��Ԫ��R�����ԭ������Ϊ________��RԪ��������_______��
(4)���a gij�����к��еķ�����Ϊb����c g�������ڱ�״���µ������(��NAΪ�����ӵ�����)______________��
(5)��CH4��O2����ɵĻ�����壬�ڱ���µ��ܶ���H2��14.5������û��������CH4��O2�������Ϊ_______��
(6)���ʵ���Ũ����ͬ��NaCl��MgCl2��AlCl3������Һ������Һ�����Ϊ5��3��2ʱ��������Һ��c(Cl)֮��Ϊ____________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2016��IUPAC����117��Ԫ��ΪTs����������![]() ����ti��n����Ts��ԭ�Ӻ���������������7������˵������ȷ����
����ti��n����Ts��ԭ�Ӻ���������������7������˵������ȷ����
A.Ts�ǵ������ڵڢ�A��Ԫ��
B.Ts��ͬλ��ԭ�Ӿ�����ͬ�ĵ�����
C.Ts��ͬ��Ԫ���зǽ���������
D.������Ϊ176��Ts���ط�����![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NaClO2)����ҪƯ����̽��С�鿪չ����ʵ�飬�ش��������⣺
ʵ�����ȡNaClO2���尴ͼװ�ý�����ȡ��

��֪��NaClO2������Һ�ڵ���38��ʱ����NaClO23H2O��38-60��ʱ����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��
(1)װ��C��������___________________��
(2)B�в���ClO2�Ļ�ѧ����ʽ_________________________��
(3)װ��D�з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ____________ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ����������_______����������ӵķ�����____________��
(4)�벹���װ��D��Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ����______________����______________����______________���õ���Ʒ��
(5)�����ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NF3�������������ڳ��³�ѹ������ɫ����ζ�����壬�����ӹ�ҵ��һ�������ĵ�����ʴ�����塣�ش��������⣺
��1��NF3�ĵ���ʽΪ______��NԪ�صĻ��ϼ�Ϊ______��
��2��F2��NH3ֱ�ӷ�Ӧ����NF3�Ļ�ѧ����ʽΪ______��
��3��ʵ����ģ�ҵ�����õ������NH4HF2��NH4FHF������ȡNF3������Ϊ��NiΪ�������ϵĺϽ��ں����������������������������ķ�Ӧ��������Ϊ̼�ظ֣�����Һ�ɻ��������á�
�ٵ��ʱNF3��______�����ɣ�����������������______���ѧʽ����
�ڵ����Һ����Ni����Fe��Cu�ĵ��ʼ�NH4HF2�ȣ��ɾ��������̽��л��������ã�
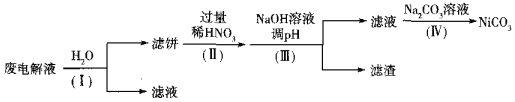
��֪��ʵ�������£����ֽ������ӿ�ʼ�����������ȫ��pH���±�
�������� | Ni2+ | Fe2+ | Cu2+ | Fe3+ |
��ʼ����ʱ��pH | 7.2 | 7.0 | 4.7 | 1.9 |
������ȫʱ��pH | 9.2 | 9.0 | 6.7 | 3.2 |
����I��Ŀ����______��������˱���Ni������������ӷ���ʽΪ______��HNO3�Ļ�ԭ����ΪNO������������pHʱ��������pHӦ���Ƶķ�Χ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���������Ȼ�ѧ����ʽ��2H2(g) + O2(g) = 2H2O(1) ��H= -571.6 kJmol-1��C3H8(g) + 5O2(g)= 3CO2(g) + 4H2O(l) ��H= -2220 kJmol-1������˵����ȷ����( )
A.��ͬ����H2��C3H8�ֱ���ȫȼ�գ�C3H8�ų���������
B.C3H8 ��ȫȼ������1mol H2O(l)�ų�������Ϊ555 kJ
C.l mol H2��2 molC3H8 ��ɵĻ��������ȫȼ�շų�������Ϊ5011.6 kJ
D.H2��C3H8�Ļ�����干4mol����ȫȼ��ʱ�ų�3256 kJ�������� n(H2)��n(C3H8)=1:1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com