|
����A��B��C��D��E������Һ�ֱ���HCl��NH3��H2O��NH4HSO4��NaOH��CH3COOH���ش��������⣺
(1)��ˮϡ��0.1 mol/LBʱ����Һ������ˮ�������Ӷ���С����(��д���)
a��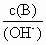
b��
c��c(H��)��c(OH��)
d��n(OH��)
(2)pH��ͬ�ĵ������������ҺA��E���ֱ���þ�۷�Ӧ����������һ����Һ�д���þ�ۣ��ҷų�������������ͬ��������˵����ȷ����________(��д���)
a����Ӧ����Ҫ��ʱ��E��A
b����ʼ��Ӧʱ������A��E
c���μӷ�Ӧ��þ�����ʵ���A��E
d��E��Һ����þ��ʣ��
(3)��������������ʵ���Ũ��B��C��Ϻ���Һ�������¶�(���ʲ���ֽ�)����ҺpH���¶ȱ仯����ͼ��________����(��д���)��

(4)�����£���0.01 mol/L��C��Һ�еμ�0.01 mol/L��D��Һ�����ԣ��õ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ________��
���±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
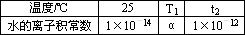
�Իش��������⣺
(1)��25��t1��t2�����________(���������������)1��10��14���������жϵ�������________��
(2)25���£�ijNa2SO4��Һ��c(SO42��)��5��10��4 mol/L��ȡ����Һ1 mL����ˮϡ����10 mL����ϡ�ͺ���Һ��c(Na+)��c(OH��)��________����pH��9��NaOH��Һ��pH��4��H2SO4��Һ��ϣ������û����ҺpH��7����NaOH��Һ��H2SO4��Һ�������Ϊ________��
(3)FeCl3��ˮ��Һ��________(��ᡱ�����С������)�ԣ�t2��ʱ��pH________6(���������������������)��ԭ����(�����ӷ���ʽ��ʾ)��________��ʵ����������FeCl3����Һʱ������FeCl3����������________�У�Ȼ����������ˮϡ�͵������Ũ�ȣ���________(��ٽ����������ơ�)��ˮ�⣬����õ����ǻ��ǵ���Һ��
| 
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�