
(1)Ļņ»ģÓŠBaCO3³ĮµķµÄNaOHČÜŅŗÖŠµĪ¼ÓŃĪĖį£¬ĪŖŹ²Ć“²»»įŹ¹BaCO3Čܽā¶ųÄܲā¶ØNaOHµÄŗ¬Įæ____________”£
(2)ĪŖŹ²Ć“ŌŚµĪ¶Ø¹ż³ĢÖŠŅŖ²»¶ĻÕńµ“׶ŠĪĘæ£æ_______________________________________”£
(3)µĪ¶ØÖÕµćŹ±ČÜŅŗŃÕÉ«ČēŗĪĶ»±ä£æ______________________________________________”£
(4)ÄÜ·ńøÄÓĆ¼×»ł³Č×÷ÖøŹ¾¼Į£¬²ā³öµÄNaOHÖŹĮæ·ÖŹżČēŗĪ£æ________________________”£
 ³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011Äźøßæ¼»Æѧ×Üø“Ļ°30·ÖÖÓĻŽŹ±ŃµĮ·£ŗ×ØĢā12””»ÆѧÓė×ŌȻ׏Ō“µÄæŖ·¢ĄūÓĆ ĢāŠĶ£ŗ058
ŗ£ŃóÖŠŗ¬ÓŠ·įø»µÄ׏Ō“£¬ŹĒČĖĄąµÄ¾Ž“ó±¦æā£®ĪŅ¹śÓµÓŠŗܳ¤µÄŗ£°¶Ļߣ¬¶«²æŃŲ°¶ŗ£Ģ²Ę½»ŗ£¬¶ąĻøɳ£¬ČÕÕÕŹ±¼ä³¤£¬²»ÉŁŃĪ³”¾ł¼ÆÖŠÓŚ“Ė£®»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÖĘŗ£ŃĪ·½·ØČēĻĀ£ŗŗ£Ė®ÕĒ³±Ź±£¬½«ŗ£Ė®ŅżČėŃĪĢļ£¬µČĀä³±Ź±£¬ŌŁÓĆČÕɹµÄ·½·Ø£¬“żŃĪĢļĖ®·ÖÕō·¢µ½Ņ»¶Ø³Ģ¶ČŹ±£¬ŌŁČöČėŹ³ŃĪæÅĮ£(³ĘĪŖ¾§ÖÖ)£¬Ź³ŃĪ¾§Ģ弓“Ó±„ŗĶČÜŅŗÖŠĪö³ö£®ĒėĖµĆ÷ŃĪĢļ»ńµĆŹ³ŃĪ¾§ĢåµÄĢõ¼ž________£®
(2)ŅŌŹ³ŃĪĪŖÖ÷ŅŖŌĮĻæÉŅŌÖʱø“æ¼ī£®ŹŌÓĆ»Æѧ·½³ĢŹ½±ķŹ¾ĘäÖʱø¹ż³Ģ£ŗ
________£®
“Ė·ØÖʵƵēæ¼īÖŠ³£ŗ¬ÓŠÉŁĮæĀČ»ÆÄĘ£¬ŅŖ²ā¶Øѳʷ֊µÄ“æ¼īµÄÖŹĮæ·ÖŹż£¬»¹æɲÉÓĆĻĀĶ¼ÖŠµÄ×°ÖĆ½ųŠŠŹµŃé£ŗ
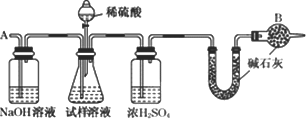
ĒėÄćÓĆŅ»¾ä»°ŠšŹöøĆŹµŃéÉč¼ĘµÄ»ł±¾ŌĄķ£ŗ________£®
(3)ĀČ¼ī»Æ¹¤³§ÖʵƵÄÉÕ¼īÖŠŅ²ŗ¬ÓŠÉŁĮæĀČ»ÆÄĘ(¼ŁÉč²»ŗ¬ĘäĖūŌÓÖŹ)£¬ÓĆÖŠŗĶµĪ¶Ø·ØÉč¼ĘŅ»øöŹµŃé·½°øŅŌ²ā¶Øѳʷ֊ÉÕ¼īŗĶĀČ»ÆÄʵÄÖŹĮæ·ÖŹż£®ŹŌ¼ĮŹĒ0.100 0 mol/LŃĪĖį”¢·ÓĢŖČÜŅŗ£¬ŅĒĘ÷ŹĒ50 mLµÄĖįŹ½µĪ¶Ø¹Ü”¢×¶ŠĪĘ森Ę䏵ŃéµÄÖ÷ŅŖ²½ÖčŹĒ________”¢________”¢µĪ¶Ø£®
(4)¹¤ŅµÉĻÓƵē½ā·ØÖʱø½šŹōÄĘ£¬µ«Öʱø½šŹō¼ŲŅ»°ć²»²ÉÓƵē½āČŪČŚĀČ»Æ¼Ų£¬¶ųŹĒ²ÉÓĆČČ»¹Ō·ØĄ“Öʱø£®ŌŚ850”ęÓĆ½šŹōÄĘĄ“»¹ŌKCl·“Ó¦ČēĻĀ£ŗ
Na(Ņŗ)£«KCl(Ņŗ)![]() NaCl(Ņŗ)£«K(Ęų)£®
NaCl(Ņŗ)£«K(Ęų)£®
¹¤ŅµÉĻÓ¦²ÉÓĆÄÄŠ©“ėŹ©²ÅÄÜŹ¹·“Ó¦ĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£æ
________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÄ£ÄāĢā ĢāŠĶ£ŗĢīæÕĢā

 NaCl(l)+ K(g)”£¹¤ŅµÉĻÓ¦²ÉÓĆÄÄŠ©“ėŹ©²ÅÄÜŹ¹·“Ó¦ĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£æ___________________
NaCl(l)+ K(g)”£¹¤ŅµÉĻÓ¦²ÉÓĆÄÄŠ©“ėŹ©²ÅÄÜŹ¹·“Ó¦ĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£æ___________________ ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com