
���� ��1����PM2.5ָ���ǿ����еķ۳���
a��ȼ���̻��������ڻ�ҩ��ը�����������̳���
b��Ϊ�������ɵ���ˮ����ʹ�����еķ۳���ˮ������������棻
c��¶�������Ҷ�����û����ȫȼ�յ��̳���
��a����Ƽ���Ҫ��������֧����������
b������ҩ��Ҫ��������θ����ࣻ
c����˾ƥ����Ҫ�������������Ը�ð����Ľ�����ʹ��
��ά����C���߲�ˮ������ǿ�˵���������Ҫ��������ά����C�Ļ�ԭ�ԣ�
��2���ٸ��������ڳ�ʪ�����з����绯ѧ��ʴ��Fe��������ʧȥ���ӷ���������Ӧ��
����֬��������ˮ�����ղ����Ǹ�֬������ͣ���������ø��������ˮ�����ɰ����
���洬��Я���ĵ�ˮʱ����˻���������ͽ��壬��Ҫ�������������������˴������н϶�ĸơ�þ��̼���Σ���֮ΪӲˮ��ͨ�������ӽ����ķ�������������������������ˮ��ȱ��������Ҫ�Ŀ����ʣ�
��3������������ЧӦ����Ҫ�����Ƕ�����̼��
�ڶ�����̼����̼��������̼�����Σ�
�۸���Һ̬CO2�ܶȴ��ں�ˮ�ܶȼ���С������CO2��Ũ�ȿ�ʹ��̬CO2��ΪҺ̬����������ȡ�Ĵ�ʩ��
��� �⣺��1����PM2.5ָ���ǿ����еķ۳�
a��ȼ���̻��������ڻ�ҩ��ը�����������̳�����a��ѡ��
b��Ϊ�������ɵ���ˮ����ʹ�����еķ۳���ˮ������������棬��bѡ��
c��¶�������Ҷ�����û����ȫȼ�յ��̳�����c��ѡ��
��ѡ��b��
��a����Ƽ���Ҫ��������֧����������
b������ҩ��Ҫ��������θ����ࣻ
c����˾ƥ����Ҫ�������������Ը�ð����Ľ�����ʹ��
��ѡ��c��
��ά����C���߲�ˮ������ǿ�˵���������Ҫ��������ά����C�Ļ�ԭ�ԣ�
��ѡ��a��
��2���ٸ��������ڳ�ʪ�����з����绯ѧ��ʴ��Fe��������ʧȥ���ӷ���������Ӧ���为���ĵ缫��Ӧʽ��Fe-2e-=Fe2+��
�ʴ�Ϊ���绯ѧ��Fe-2e-=Fe2+��
����֬��������ˮ�����ղ����Ǹ�֬������ͣ���������ø��������ˮ�����ɰ����ᣬ�ʴ�Ϊ����֬��������
���洬��Я���ĵ�ˮʱ����˻���������ͽ��壬��Ҫ�������������������˴������н϶�ĸơ�þ��̼���Σ���֮ΪӲˮ��ͨ�������ӽ����ķ�������������������������ˮ��ȱ��������Ҫ�Ŀ����ʣ��ʴ�Ϊ�����ˣ�Ӳˮ�����ӽ�����������������ˮ��ȱ��������Ҫ�Ŀ����ʣ�
��3���ٻ�ʯȼ�ϵĴ���ʹ�ã�ʹ�ÿ������������������̼�����������ߣ������������ߣ�
�ʴ�Ϊ������ȼ��ú��ʯ�͵ȿ���ȼ�ϣ�
�ڴ���ɺ��������Ҫ�ɷ���CaCO3������ˮ��ʴ��������̼����CaCO3����̼����ƣ���ѧ����ʽΪ��CaCO3+H2O+CO2=Ca��HCO3��2���ʴ�Ϊ��CaCO3+H2O+CO2=Ca��HCO3��2��
�������ʵ���̬�仯��֪���ڴ��������£�������̼����ɫ��ζ�����壬�����¶ȵ���31.2��ʱ����ѹ��ʹCO2��ΪҺ̬������ѹ���¿ɼ�С����֮��ļ����ʹ������̼�������ΪҺ�壬��ɽ�CO2Һ������������ף��Լ�С������CO2��Ũ�ȣ���ѡD��
���� ������Ҫ�����˻�����ҩ�Ӫ�����ʵ���������صĻ�ѧ���⣬ƽʱע�����һЩ���ʶ������ⲻ�ѣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
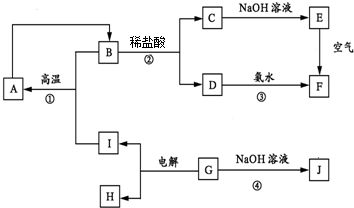
 ��E�Ļ�ѧʽΪFe��OH��2��
��E�Ļ�ѧʽΪFe��OH��2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢ� | B�� | �ۢ� | C�� | �٢ڢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��2��3 | B�� | 6��3��2 | C�� | 3��2��1 | D�� | 1��1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ӱ뾶 Z��X | B�� | ���������� Z��Y | ||
| C�� | ������ Z��X | D�� | ԭ�Ӱ뾶 Z��Y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ȡ����Ӧ��
��ȡ����Ӧ�� ��ȡ����Ӧ��
��ȡ����Ӧ�� ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ�� CH3CH2OOCCOOCH2CH3+2H2O��������Ӧ��
CH3CH2OOCCOOCH2CH3+2H2O��������Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4+2NaOH�TCu��OH��2��+Na2SO4 | B�� | Cl2+H2O?HCl+HClO | ||
| C�� | Zn+H2SO4�TZnSO4+H2�� | D�� | 2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� | 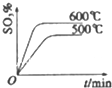 | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ȵ�����ˮ���� | B�� | ����ϡ���ᷴӦ | ||
| C�� | ����NaOH��Һ��Ӧ | D�� | ����ˮ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com