
ŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£ŅŃÖŖNaAlO2+CO2+H2O=Al(OH)3”ż+NaHCO3£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ
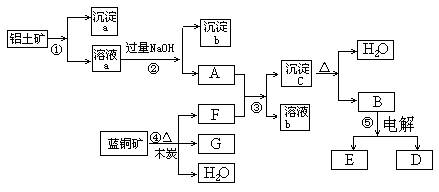
(1) Š“³ö¢ŚµÄĄė×Ó·½³ĢŹ½£ŗ______________________”¢_____________________”£
(2) ³Įµķa”¢c»Æѧ³É·Ö·Ö±šŹĒ£ŗ””___ ___”¢__ ____”£””””
(3)ĒėŠ“³ö¼ģŃé³ĮµķbÖŠĖłŗ¬ÓŠŃōĄė×ӵďµŃé·½·Ø_______________________________
(4)Ļ“µÓ³ĮµķcµÄŹµŃé²Ł×÷·½·ØŹĒ____________________________________________
¼ÓČČ³ĮµķcÓ¦·ÅŌŚ___________ČŻĘ÷ÖŠ½ųŠŠ”£
(5) ¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______________________”¢_________ ___________________________________”£
(1) Al3++4OH”Ŗ= AlO2”Ŗ+2H2O Fe3++3OH”Ŗ=Fe£ØOH£©3
(2) SiO2 Al£ØOH£©3
(3) Č”³ĮµķÉŁĮæÓŚŅ»Ö§½ą¾»µÄŹŌ¹ÜÖŠ£¬¼ÓČėÉŁĮæŃĪĖį£¬Č»ŗóŌŁĶłŹŌ¹ÜÖŠ¼ÓČė¼øµĪµÄKSCNČÜŅŗ£¬·¢ĻÖŹŌ¹ÜÄŚ³ŹĻÖŗģÉ«”£
(4) ŌŚĀ©¶·ÄŚµÄ³ĮµķÉĻ¼ÓČėŅ»¶ØĮæµÄÕōĮóĖ®£¬Ć»¹ż³Įµķ£¬ŹĢĖ®×ŌČ»Į÷ĻĀ£¬ÖŲø“Źż“Ī£»ŪįŪö
£Ø5£©2 (2CuCO3”¤Cu(OH)2)+3C![]() 6Cu+ 7CO2”ü+2H2O 2Al2O3
6Cu+ 7CO2ӟ+2H2O 2Al2O3![]() 4Al+3O2ӟ
4Al+3O2ӟ
½įŗĻĢāÄæŠÅĻ¢¼°æņĶ¼æÉÖŖ£ŗ¢Ł¼ÓČėµÄĪŖ×ćĮæµÄŃĪĖį»ņĮņĖį£¬³ĮµķaĪŖ¶žŃõ»Æ¹č£¬ČÜŅŗaĪŖĢśŗĶĀĮµÄŃĪČÜŅŗ£¬³ĮµķbĪŖĒāŃõ»ÆĢś£»½įŗĻæņĶ¼æɵĆFĪŖ¶žŃõ»ÆĢ¼£¬GĪŖĶ£¬³ĮµķcĪŖĒāŃõ»ÆĀĮ£¬BĪŖŃõ»ÆĀĮ”£


 ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ½š×“ŌŖ¼ØÓÅŗĆ¾ķĻµĮŠ“š°ø
½š×“ŌŖ¼ØÓÅŗĆ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09-10ğƷ֯ŹŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©ŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£¾¹żŅ»¶ØĢõ¼žµÄ×Ŗ»Æ¶žÕß¾łæÉ×Ŗ»ÆĪŖ½šŹōµ„ÖŹ£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ
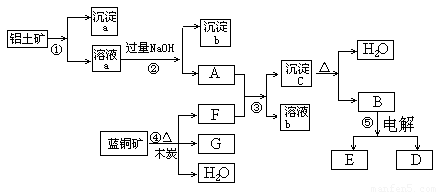
(1)Š“³ö¢ŁµÄĄė×Ó·½³ĢŹ½£ŗ______________________”¢_____________________”£
(2)¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
______________ _______Ӣ___________ ___________ӣ
(3)Čō¹żĮæFÓėA·“Ó¦£¬ČÜŅŗbµÄ³É·ÖŹĒ£ŗ__ ____£ØŠ“»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£ŅŃÖŖNaAlO2+CO2+H2O=Al(OH)3”ż+NaHCO3£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ
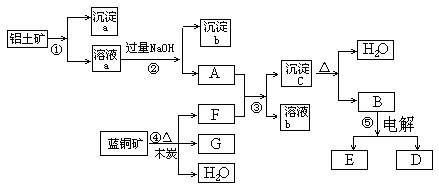
(1) Š“³ö¢ŚµÄĄė×Ó·½³ĢŹ½£ŗ______________________”¢_____________________”£
(2) ³Įµķa”¢c»Æѧ³É·Ö·Ö±šŹĒ£ŗ””___ ___”¢__ ____”£””””
£Ø3£©ĒėŠ“³ö¼ģŃé³ĮµķbÖŠĖłŗ¬ÓŠŃōĄė×ӵďµŃé·½·Ø_______________________________
£Ø4£©Ļ“µÓ³ĮµķcµÄŹµŃé²Ł×÷·½·ØŹĒ____________________________________________
¼ÓČČ³ĮµķcÓ¦·ÅŌŚ___________ČŻĘ÷ÖŠ½ųŠŠ”£
(5) ¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______________________”¢_________ ___________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£¾¹żŅ»¶ØĢõ¼žµÄ×Ŗ»Æ¶žÕß¾łæÉ×Ŗ»ÆĪŖ½šŹōµ„ÖŹ£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ
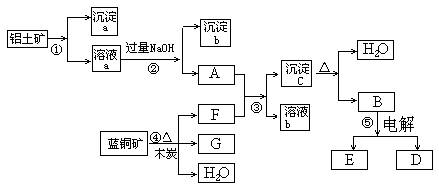
(1)Š“³ö¢ŁµÄĄė×Ó·½³ĢŹ½£ŗ______________________”¢_____________________”£
(2)¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
______________ _______Ӣ___________ ___________ӣ
(3)Čō¹żĮæFÓėA·“Ó¦£¬ČÜŅŗbµÄ³É·ÖŹĒ£ŗ__ ____£ØŠ“»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09”Ŗ10ğƷ֯ŹŠŌųĻÜč÷֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©ŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£¾¹żŅ»¶ØĢõ¼žµÄ×Ŗ»Æ¶žÕß¾łæÉ×Ŗ»ÆĪŖ½šŹōµ„ÖŹ£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ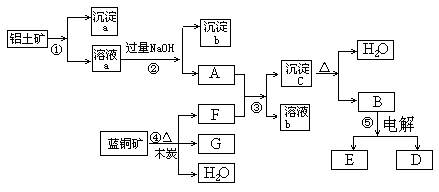
(1)Š“³ö¢ŁµÄĄė×Ó·½³ĢŹ½£ŗ______________________”¢_____________________”£
(2)¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
______________ _______Ӣ___________ ___________ӣ
(3)Čō¹żĮæFÓėA·“Ó¦£¬ČÜŅŗbµÄ³É·ÖŹĒ£ŗ__ ____£ØŠ“»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com