
| ���� N��mol�� ʱ�䣨min�� | CO2 | H2 | CaO |
| 0 | 0.50 | 1.00 | 0.40 |
| 2 | 0.35 | 0.60 | a |
| 6 | 0.20 | 0.20 | b |
| 8 | 0.20 | 0.20 | b |
���� ��1����CO��g��+H2O��g��?CO2��g��+H2��g����H=-41kJ•mol-1
��CO2��g��+4H2��g��?CH4��g��+2H2O��g����H=-187kJ•mol-1����+�ڵ�CO��g��+3H2��g��?CH4��g��+H2O��g����H=-228kJ•mol-1�ݴ˽��з�����
��2���پ�v=$\frac{��c}{��t}$���м��㣻
��CaO��s��+CO2��g��?CaCO3��s���ݴ˷���ʽ����CaO��s����CO2��g��ǰϵ����Ϊ1��0-6min��CO2��g�������ʵ����仯��0.30mol����CaO��s�������ʵ����仯ҲΪ0.30mol���ݴ˽��з�����
��A����������������൱�ڼ�Сѹǿ���������������ķ�����У��������������n��H2�������ǵò���ʧ��c��H2����С��
B�������¶ȣ������ȷ�����У������淴Ӧ������У���n��CO2������
C����������H2��ʹ��ϵѹǿ���������������С�ķ�����У���������Ӧ������У�
D����������CO2��ʹ��ϵѹǿ���������������С�ķ�����У���������Ӧ������У���c��CH4������
�ܾ�k=$\frac{c��C{H}_{4}��{c}^{2}��{H}_{2}O��}{c��C{O}_{2}��{c}^{4}��{H}_{2}��}$�˽��м��㣻���QC��ȷ����ķ�Χ��
��3������ȼ�ϵ�أ��ܵĵ缫��Ӧ����ʽΪCH4+3O2�TCO2+2H2O��
A��CH4+2O2�TCO2+2H2O��8mole-
22.4��3 8
3.36 0.4���ʷ�Ӧ�����й�ת�Ƶ���0.4mol��
B��CH4+2O2�TCO2+2H2O��8mole-
22.4��3 1
3.36 0.05����n��CO2��=0.05mol��n��NaOH��=0.05L��2mol•L-1=0.1mol���ݴ�ȷ����Һ�ɷ֣�
C����һ��������Na2CO3��Һ����ˮ��ƽ�⣺CO32-+H2O?HCO3-+OH-����Һ�Լ��ԣ�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
D�����������غ��2c��CO32-��+2c��HCO3-��+2c��H2CO3��=c��Na+�������ݵ���غ��c��Na+��+c��H+ ��=c��OH-��+c��HCO3- ��+2c��CO32-�����ݴ˽��з�����
��� �⣺��1����CO��g��+H2O��g��?CO2��g��+H2��g����H=-41kJ•mol-1
��CO2��g��+4H2��g��?CH4��g��+2H2O��g����H=-187kJ•mol-1����+�ڵ�CO��g��+3H2��g��?CH4��g��+H2O��g����H=-228kJ•mol-1��
�ʴ�Ϊ��CO��g��+3H2��g��?CH4��g��+H2O��g����H=-228kJ•mol-1��
��2����v��H2��=$\frac{��c}{��t}$=$\frac{\frac{0.4mol}{10L}}{2min}$=0.02mol/L��min��
�ʴ�Ϊ��0.02mol/L��min��
��CaO��s��+CO2��g��?CaCO3��s���ݴ˷���ʽ����CaO��s����CO2��g��ǰϵ����Ϊ1��0-6min��CO2��g�������ʵ����仯��0.30mol����CaO��s�������ʵ����仯ҲΪ0.30mol����b=0.40mol-0.30mol=0.10mol��
�ʴ�Ϊ��0.10��
��A����������������൱�ڼ�Сѹǿ���������������ķ�����У��������������n��H2�������ǵò���ʧ��c��H2����С����A����
B�������¶ȣ������ȷ�����У������淴Ӧ������У���n��CO2������B��ȷ��
C����������H2��ʹ��ϵѹǿ���������������С�ķ�����У���������Ӧ������У���CaCO3�������ӣ���C ����
D����������CO2��ʹ��ϵѹǿ���������������С�ķ�����У���������Ӧ������У���c��CH4������D��ȷ��
�ʴ�Ϊ��AC��
��CO2��g��+4H2��g��?CH4��g��+2H2O��g��
��ʼ��c�� 0.05 0.1 0 0
�仯 0.02 0.08 0.02 0.04
ƽ�� 0.03 0.02 0.02 0.04
�ʻ�ѧƽ�ⳣ��k=$\frac{c��C{H}_{4}��{c}^{2}��{H}_{2}O��}{c��C{O}_{2}��{c}^{4}��{H}_{2}��}$=$\frac{0.02��0.0{4}^{2}}{0.03��0.0{2}^{4}}$=$\frac{2}{3}$��Ҫʹv����CO2����v�棨CO2������Ӧ������Ӧ�������QC=$\frac{0.1��0��{1}^{2}}{0.1��{x}^{4}}$$��\frac{2}{3}$����0��x$��\root{4}{0.015}$��
�ʴ�Ϊ��$\frac{2}{3}$��0��x$��\root{4}{0.015}$��
��3������ȼ�ϵ�أ��ܵĵ缫��Ӧ����ʽΪCH4+3O2�TCO2+2H2O��
A��CH4+2O2�TCO2+2H2O��8mole-
22.4��3 8
3.36 0.4���ʷ�Ӧ�����й�ת�Ƶ���0.4mol����A����
B��CH4+2O2�TCO2+2H2O��8mole-
22.4��3 1
3.36 0.05����n��CO2��=0.05mol��n��NaOH��=0.05L��2mol•L-1=0.1mol����n��NaOH����n��CO2��=2��1��
2 NaOH+CO2=Na2CO3+H2O���ɷ���ʽ�ã�n��NaOH����n��CO2��=2��1������ҺΪNa2CO3����B����
C����һ��������Na2CO3��Һ����ˮ��ƽ�⣺CO32-+H2O?HCO3-+OH-����Һ�Լ��ԣ�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������C��ȷ��
D�����������غ��2c��CO32-��+2c��HCO3-��+2c��H2CO3��=c��Na+���٣����ݵ���غ��c��Na+��+c��H+ ��=c��OH-��+c��HCO3- ��+2c��CO32-���ڣ��ɢ٢������ã�c��OH-��=2c��H2CO3��+c��HCO3- ��+c��H+ ������D��ȷ��
�ʴ�Ϊ��CD��
���� ������Ҫ�����Ȼ�ѧ����ʽ����д����ѧ��Ӧ���ʼ�ƽ�ⳣ���ļ��㣬ƽ���ƶ���Ӱ�����صȣ�������Ŀ�Ѷ��еȣ�


 ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H2 | B�� | CO2 | C�� | BeCl2 | D�� | SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaH��ˮ�������� | |
| B�� | NaH�������Ӱ뾶������Ӱ뾶�� | |
| C�� | NaH�������ӿɱ���ԭΪ���� | |
| D�� | Na����������������뱣����ú���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2��CO2��Ӧ����Na2CO3��Na2O2+CO2�TNa2CO3+O2 | |
| B�� | Na2CO3��Һ�ʼ��ԣ�CO32-+H2O?H2CO3+OH- | |
| C�� | Na2CO3��Һ��ȥCH3COOC2H5�е�CH3COOH��CO32-+2H+�TCO2��+H2O | |
| D�� | ����Na2CO3��Һ����ˮ���е�CaSO4��CO32-+CaSO4�TCaCO3+SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3 | B�� | Na2O | C�� | NaOH | D�� | Na2CO3•10H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
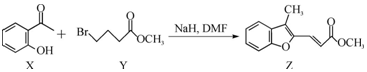
| A�� | ����X������̼ԭ�ӿ�����ͬһƽ���� | |
| B�� | ����FeCl3��Һ����Z���Ƿ���X | |
| C�� | �����ʵ�����X��Z �ֱ���H2�ӳɣ��������H2�����ʵ���֮��Ϊ3��5 | |
| D�� | �����ʵ�����X��Y�ֱ���NaOH��Ӧ���������NaOH�����ʵ���֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������仯�����ת������Դ���úͻ�����������Ҫ�о����⣮
�������仯�����ת������Դ���úͻ�����������Ҫ�о����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�����������ӡ����ӡ�������ɵ� | |
| B�� | ������һ����ͬ����������������һ������ͬ | |
| C�� | �˵�����ͺ��������һ����� | |
| D�� | ��ѧ���ʼ�����ȫ��ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cn��H2O��m | B�� | ��C2O3��n��H2O��m | C�� | ��C2H��n��H2O��m | D�� | ��CO��n��H2O��m |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com