
��18�֣�ijͬѧ���������װ��������ȡSO2����֤SO2�����ʡ�����©����װ75%��Ũ���ᣬ��ƿ��װ����Na2SO3���Իش��������⣺
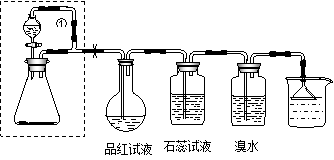
����ƿ�ڷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��ʵ������У�Ʒ����Һ����ɫ��Ϊ�� ��ʯ����Һ����ɫ�仯Ϊ�� ��ʵ��������ƿ��Ʒ����Һ����ɫ�仯Ϊ�� ��
����ˮ��SO2��Ӧ�����ӷ���ʽΪ�� ��
�ȵ��ܢٵ������ǣ� ������©���������� ��
�ɷ�����װ���ܷ����շ������� ���ش��ܡ����ܡ�����
��Na2SO3+H2SO4��Na2SO4+SO2��+H2O��2�֣� ���ɺ�ɫ��Ϊ��ɫ��2�֣���������ɫ��Ϊ��ɫ������ɫ����ȥ��2�֣�����ɫ�ָֻ�Ϊԭ���ĺ�ɫ��2�֣���Br2+SO2+H2O��4H++2Br-+SO42-��3�֣���ƽ���Һ©������ƿ��ѹǿ��ʹҺ���ܹ�˳�����£�3�֣�����ֹ�ձ���Һ�嵹��������Լ�ƿ�� ��2�֣��ɲ��ܣ�2�֣���
����ּ�ڿ���SO2�����ʼ���ʵ����Ƶļ���������ۡ��ŢǶ�Ϊ�����Ļ�ѧ��Ӧ����Լ�������SO2����Ư��������Ư�����ȶ��ԣ���Ʒ���ȱ���ɫ�����Ⱥ����ָ�Ϊ��ɫ������SO2����ʹָʾ����ɫ��������Һֻ�ܱ��ֳ����Զ�ʹʯ���Ϊ��ɫ���ȵ������Ҳ�Ķ��װ�û��SO2����������谭���ã�����ƿ�ڵ�ѹǿ�ϴ�Һ�岻�����¡��ɴ˿����뵽���ܢٵ�������Ϊƽ��ѹǿ֮���á���һ����Na2SO3�ľ�����С�����������ᣬ��һ����Na2SO3������ˮ���������������ܽ⣬ʹ��Ӧʧȥ���ƣ��ʲ���ʹ�����շ�������
��������ʾ��I.SO2��Ư����ѡ���ԣ�������ʹָʾ����ɫ������ֻ��ʹʯ������ɫ��Ϊ��ɫ ��.���շ�����ֻ��ͬʱ�߱���������ʱ����ʹ�ã��ٷ�Ӧ����Ҫ���ȣ��ڷ�Ӧ����ų��������ȣ��۷�ӦҩƷΪ�����Һ�壬�ҹ���ҩƷΪ��״������Һ��ˮ��


 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����NaHCO3��Na2CO3?xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ���������װ�ý���ʵ�飺
����NaHCO3��Na2CO3?xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ���������װ�ý���ʵ�飺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺
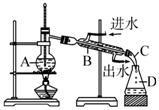
(1)ָ��ʵ��װ��������A��B��C��D�����ơ�
(2)ָ����ͬѧ����Ƶ�ʵ��װ���д��ڵĴ������������
(3)ʵ��װ�ø�������ν��������Լ�飿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�챱���и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
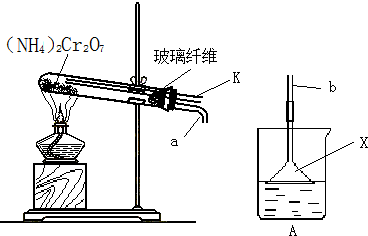
��̽��������ⵥ�ʵ�������ǿ����ijͬѧ���������װ�ã���Ũ�����KMnO4���巴Ӧ��ȡ��������
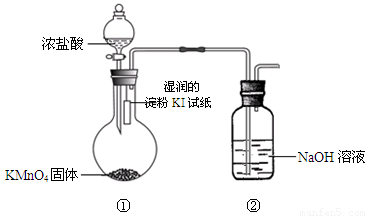
��ش�
��1��ʵ���й۲쵽ʪ��ĵ���KI��ֽ ��д�����з������û���Ӧ�����ӷ���ʽ�� ��
��2��ʵ����ۣ��ȵ��ʵķǽ����Աȵⵥ�ʵ� ���ǿ��������������ԭ�ӽṹ�ǶȽ��ͣ��Ⱥ͵�λ�����ڱ��� �壬����Ԫ�ش��ϵ��£� ���õ�������������
��3��װ�âڵ������� ��
д����Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com