
| A£® | ¢Ł¢Ś¢Ū¢Ž | B£® | ¢Ł¢Ś¢Ü | C£® | ¢Ū¢Ż¢Ž | D£® | ¢Ś¢Ū¢Ż |
·ÖĪö ¢Łµ„Ō×Ó·Ö×Ó²»“ęŌŚ¹²¼Ū¼ü£»
¢Ś¾§ĢåÖŠÖ»ŅŖÓŠŅõĄė×Ó¾ĶŅ»¶ØÓŠŃōĄė×Ó£»
¢Ūøł¾Ż¾§ĢåĄąŠĶÅŠ¶ĻČŪµćøßµĶ£»
¢ÜĄė×Ó¾§ĢåÖŠŅ»¶Ø“ęŌŚĄė×Ó¼ü£¬µ«²»Ņ»¶Øŗ¬ÓŠ½šŹōŌŖĖŲ£»
¢Żøł¾ŻŅõŃōĄė×ÓĖł“ųµēŗÉŹżŌ½¶ą£¬Ąė×Ó°ė¾¶Ō½Š”£¬¾§øńÄÜŌ½“ó£»
¢ŽNaCl¾§°ū½į¹¹ĪŖ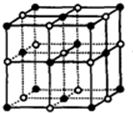 £¬¾§ĢåÖŠÓėĆæøöNa+¾ąĄėĻąµČĒŅ×ī½üµÄNa+øöŹż=3”Į8”Ā2£®
£¬¾§ĢåÖŠÓėĆæøöNa+¾ąĄėĻąµČĒŅ×ī½üµÄNa+øöŹż=3”Į8”Ā2£®
½ā“š ½ā£ŗ¢Łµ„Ō×Ó·Ö×Ó²»“ęŌŚ¹²¼Ū¼ü£¬Ö»“ęŌŚ·Ö×Ó¼ä×÷ÓĆĮ¦£¬¹Ź¢Ł“ķĪó£»
¢Ś¾§ĢåÖŠÖ»ŅŖÓŠŅõĄė×Ó¾ĶŅ»¶ØÓŠŃōĄė×Ó£¬¹Ź¢ŚÕżČ·£»
¢Ū¾§ĢåÖŠČŪµćøßµĶŅ»°ćĖ³ŠņŹĒ£ŗŌ×Ó¾§Ģ壾Ąė×Ó¾§Ģ壾·Ö×Ó¾§Ģ壻ŌŚŌ×Ó¾§ĢåÖŠ£¬Ō×Ó°ė¾¶Ō½“óČŪµćŌ½µĶ£»ŌŚĄė×Ó¾§ĢåÖŠ£¬Ąė×Ó°ė¾¶Ō½“ó£¬ČŪµćŌ½µĶ£¬µēŗÉŌ½¶ą£¬ČŪµćŌ½øߣ»ŌŚ·Ö×Ó¾§ĢåÖŠ£¬ĪļÖŹµÄČŪµćÓėĻą¶Ō·Ö×ÓÖŹĮæ³ÉÕż±Č£Øŗ¬ÓŠĒā¼üµÄĪļÖŹ³żĶā£©£¬ĖłŅŌÕā¼øÖÖĪļÖŹµÄČŪµćøßµĶĖ³ŠņŹĒ£ŗ½šøÕŹÆ”¢SiC”¢NaF”¢NaCl”¢H2O”¢H2S¾§ĢåµÄČŪµćŅĄ“Ī½µµĶ£¬¹Ź¢ŪÕżČ·£»
¢ÜĄė×Ó¾§ĢåÖŠŅ»¶Ø“ęŌŚĄė×Ó¼ü£¬µ«²»Ņ»¶Øŗ¬ÓŠ½šŹōŌŖĖŲ£¬ČēĀČ»Æļ§²»ŗ¬½šŹōµÄĄė×Ó»ÆŗĻĪļ£¬¹Ź¢Ü“ķĪó£»
¢ŻF-”¢Cl-”¢Br-µÄĄė×Ó°ė¾¶Öš½„Ōö“ó£¬ĖłŅŌ¾§øńÄÜÓɓ󵽊”Ė³Šņ£ŗNaF£¾NaCl£¾NaBr£¬ÓÖMgOĖł“ųµēŗÉŹż¶ą£¬ĖłŅŌ¾§øńÄÜ×ī“󣬹Ź¢ŻÕżČ·£»
¢ŽNaCl¾§°ū½į¹¹ĪŖ £¬NaCl¾§ĢåÖŠÓėĆæøöNa+¾ąĄėĻąµČĒŅ×ī½üµÄNa+øöŹż=3”Į8”Ā2=12£¬¹Ź¢Ž“ķĪó£»
£¬NaCl¾§ĢåÖŠÓėĆæøöNa+¾ąĄėĻąµČĒŅ×ī½üµÄNa+øöŹż=3”Į8”Ā2=12£¬¹Ź¢Ž“ķĪó£»
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éµÄÖŖŹ¶µć½Ļ¶ą£¬Éę¼°¹²¼Ū¼ü”¢Ąė×Ó¼ü”¢·Ö×Ó¼ä×÷ÓĆĮ¦”¢¾§ĢåČŪµć±Č½ĻµČ£¬ĢāÄæ²ąÖŲÓŚ»ł“”ÖŖŹ¶µÄ漲飬ÄŃ¶Č²»“ó£¬×¢ŅāĻą¹ŲÖŖŹ¶µÄ»żĄŪ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ӣ
”¢ ½į¹¹·Ē³£²»ĪČ¶Ø£¬»įŃøĖŁ×Ŗ±äĪŖ
½į¹¹·Ē³£²»ĪČ¶Ø£¬»įŃøĖŁ×Ŗ±äĪŖ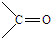
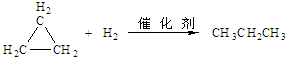
 £®
£®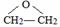 £®
£®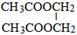 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÖŲ¾§ŹÆ BaSO4 ±µ²Ķ | B£® | Ć÷·Æ KAlSO4•12H2O ¾»Ė®¼Į | ||
| C£® | ĀĢ·Æ Fe2£ØSO4£©3•7H2O ²¹ŃŖ¼Į | D£® | ÉśŹÆøą 2CaSO4•H2O ŹÆøą±Į“ų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
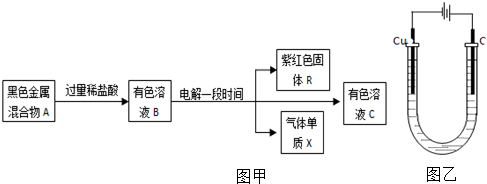
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
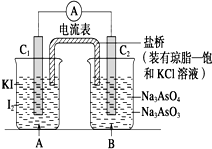 ŅŃÖŖ·“Ó¦AsO${\;}_{4}^{3-}$+2I-+2H+?AsO${\;}_{3}^{3-}$+I2+H2OŹĒæÉÄę·“Ó¦£®Éč¼ĘČēĶ¼×°ÖĆ
ŅŃÖŖ·“Ó¦AsO${\;}_{4}^{3-}$+2I-+2H+?AsO${\;}_{3}^{3-}$+I2+H2OŹĒæÉÄę·“Ó¦£®Éč¼ĘČēĶ¼×°ÖĆ| A£® | ²Ł×÷¢ń¹ż³ĢÖŠ£¬C1ĪŖÕż¼« | |
| B£® | ²Ł×÷¢ņ¹ż³ĢÖŠ£¬ŃĪĒÅÖŠµÄK+ŅĘĻņBÉÕ±ČÜŅŗ | |
| C£® | ¢ń²Ł×÷¹ż³ĢÖŠ£¬C2°ōÉĻ·¢ÉśµÄ·“Ó¦ĪŖAsO${\;}_{4}^{3-}$+2H++2e-=AsO${\;}_{3}^{3-}$+H2O | |
| D£® | ¢ņ²Ł×÷¹ż³ĢÖŠ£¬C1°ōÉĻ·¢ÉśµÄ·“Ó¦ĪŖ2I--2e-=I2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
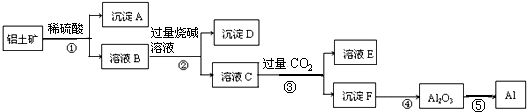
| A£® | ³ĮµķAÖ÷ŅŖŹĒSiO2 | B£® | ²½Öč¢ŚµÄÄæµÄŹĒ·ÖĄėFe3+ŗĶAl3+ | ||
| C£® | ČÜŅŗEČÜÖŹÖ÷ŅŖÓŠĢ¼ĖįÄĘŗĶĮņĖįÄĘ | D£® | ¢ŻÖ÷ŅŖŹĒ°ŃµēÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
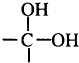 ”ś
”ś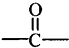 +H2OĶ¼ÖŠBŗĶ
+H2OĶ¼ÖŠBŗĶ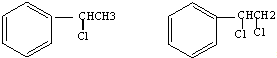 µČ¶¼ŹĒAŗĶCl2·¢Éś·“Ӧɜ³ÉµÄ²śĪļ£¬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£¬Ķø¹āŠŌÄÜŗĆ£¬³£ÓĆ×÷Ņ»Š©µĘŹĪĶāæĒ£Ø¹ż³ĢÖŠŅ»Š©Š”·Ö×ÓĪ“Š“³ö£©£®
µČ¶¼ŹĒAŗĶCl2·¢Éś·“Ӧɜ³ÉµÄ²śĪļ£¬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£¬Ķø¹āŠŌÄÜŗĆ£¬³£ÓĆ×÷Ņ»Š©µĘŹĪĶāæĒ£Ø¹ż³ĢÖŠŅ»Š©Š”·Ö×ÓĪ“Š“³ö£©£®
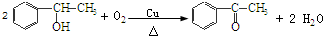 ”Æ£¬øĆ·“Ó¦ĄąŠĶĪŖŃõ»Æ·“Ó¦
”Æ£¬øĆ·“Ó¦ĄąŠĶĪŖŃõ»Æ·“Ó¦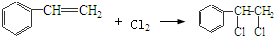 £¬øĆ·“Ó¦ĄąŠĶĪŖ¼Ó³É·“Ó¦
£¬øĆ·“Ó¦ĄąŠĶĪŖ¼Ó³É·“Ó¦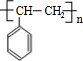
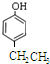 Ӣ
Ӣ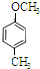 Ӣ
Ӣ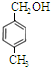
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØĮņĖįµĪŌŚÖ½ÉĻŹ¹Ö½±äŗŚ£¬ŹĒÓÉÓŚÅØĮņĖįÓŠĶŃĖ®ŠŌ | |
| B£® | Ļ”ŹĶÅØĮņĖįŹ±£¬Ó¦½«Ė®ŃŲĘ÷±ŚĀżĀż×¢ČėÅØĮņĖįÖŠ£¬²¢²»¶ĻÓĆ²£Į§°ō½Į°č | |
| C£® | Óū³żČ„CO2ÖŠµÄĖ®ÕōĘų£¬æɽ«ĘųĢåĶعżŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘæ | |
| D£® | ²»É÷ŌŚĘ¤·ōÉĻÕ“ÉĻÉŁĮæÅØĮņĖįŹ±£¬Ó¦Į¢¼“ÓĆ“óĮæĖ®³åĻ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŌ CH”ŌCH ŗĶ HCl ·“Ó¦ÖĘĀČŅŅĻ©£¬½ų¶ųÖʱø¾ŪĀČŅŅĻ©ĖÜĮĻ | |
| B£® | ÅØ°±Ė®µĪČėÉśŹÆ»ŅÖŠ£¬½«²śÉśµÄĘųĢåĶØČė AlCl3ČÜŅŗ£¬æɵƵ½ŗ¬AlO2-µÄČÜŅŗ | |
| C£® | Ź¹ÓĆ“ß»Æ¼Į²»ÄÜøı乤ŅµŗĻ³É NH3 µÄ·“Ó¦ĻŽ¶Č | |
| D£® | ÓĆ½žÓŠ KMnO4ČÜŅŗµÄ¹čĶĮĄ“ĪüŹÕĖ®¹ūŹĶ·ÅµÄŅŅĻ© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com