
 CO2(g)����H1<0��
CO2(g)����H1<0�� CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0�� 2CO2(g)����H3<0��
2CO2(g)����H3<0�� 2H2O(g)����H4<0��
2H2O(g)����H4<0�� CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1 TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1 TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=����������  2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1 Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1 Fe(s)+CO2(g)����H
Fe(s)+CO2(g)����H Fe(s)+CO2(g)����H="-218" kJ��mol-1
Fe(s)+CO2(g)����H="-218" kJ��mol-1

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2(g)+
H2(g)+  F2(g) = HF(g) ��H = ��269kJ��mol��1
F2(g) = HF(g) ��H = ��269kJ��mol��1 O2(g) = H2O(g) ��H = ��242kJ��mol��1
O2(g) = H2O(g) ��H = ��242kJ��mol��1 2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����| ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
| n(NO)(mol) | 0.200 | 0.100 | 0.080 | 0.050 | 0.050 | 0.050 |
| n(O2)(mol) | 0.100 | 0.050 | 0.040 | 0.025 | 0.025 | 0.025 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


 CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
 CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2����
CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol 2NH3(g)��Ӧ��Ӱ�졣
2NH3(g)��Ӧ��Ӱ�졣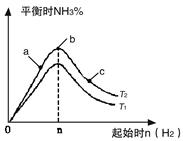
 4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������
4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������| t/s | 0 | 50 | 100 |
| c(N2O5)/mol��L��1 | 5��0 | 3��5 | 2��4 |
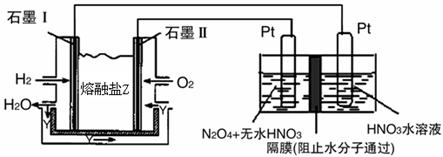
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 CO2(g)+2H2O(l)����H1=-Q1
CO2(g)+2H2O(l)����H1=-Q1 H2O(g)����H2=-Q2
H2O(g)����H2=-Q2 H2O(l)����H3=-Q3,������ȡ�����Ϊ4��1�ļ���������Ļ������11.2 L(���ۺϳɱ�״��),����ȫȼ�ջָ�������,�ų���������(����)
H2O(l)����H3=-Q3,������ȡ�����Ϊ4��1�ļ���������Ļ������11.2 L(���ۺϳɱ�״��),����ȫȼ�ջָ�������,�ų���������(����)| A��0.4Q1+0.05Q2 | B��0.4Q1+0.1Q2 |
| C��0.4Q1+0.05Q3 | D��0.4Q1+0.1Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(
2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����( )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
| HClO | HSCN | H2CO3 |
| K=3.210-8 | K=0.13 | Kl=4.210-7 K2=5.610-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(4x-y)" kJ/mol |
| B��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(2x-y)" kJ/mol |
| C��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(2y-x)" kJ/mol |
| D��2SO2(g)+2KOH(l)=2KHSO3(l)��H="-(8x-2y)" kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com