
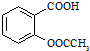
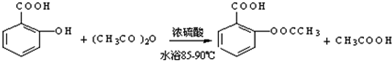
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�
 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��221 kJ |
| B��557 kJ |
| C��242 kJ |
| D��188 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����AlCl3��Һ��Al2��SO4��3��Һ�ֱ���ȡ����ɡ����գ����ù���ɷ���ͬ |
| B������FeSO4��Һʱ����FeSO4��������ϡ�����У�Ȼ��ϡ��������Ũ�� |
| C����ľ�ҿ������̬���ʻ��ʹ�� |
| D���������ƣ�Na2FeO4����Ӧ��������ˮ����������ˮ |
�鿴�𰸺ͽ���>>
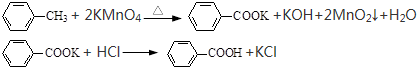
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʳ��Ϊ��������ƴ����֣����ұ��涨����ʳ�����Ậ�����õ���3.5g/100mL��
ʳ��Ϊ��������ƴ����֣����ұ��涨����ʳ�����Ậ�����õ���3.5g/100mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH3OOCCH2COOC2H5 |
| B��C2H5OOCCOOC2H5 |
| C��CH3COOCH2CH2COOCH3 |
| D��CH3COOCH2COOC2H5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com