
������������װ����֤��Ũ����������п��Ӧʱ�ɵõ�SO2��H2�������壬�����Լ���ѡ��
(1)������ķ����У�����ʵ������ʵ��Ŀ�ĵ�װ��ʾ��ͼ(����ѡ������ͼ���г�װ�á����ӽ��ܡ���Ƥ����β������װ�ò��ػ�����Ҳ���ر��װ�����Լ�����Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸA��B��C����������ѡ�õ�����(��������)���ױ�ʾ���£�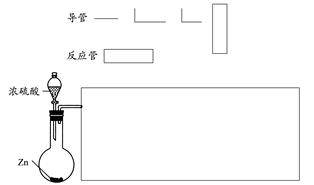
(2)���ݷ����е�װ��ͼ����д�±���
| �������� | �������������� | ���� |
| | | |
| ���һ��װ��(��ͼʱ�������) | Ũ���� | ��ֹ������ˮ��������E�и���ʵ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л������У������ɵ���ֱ�ӻ����Ƶã������ɸ��ֽⷴӦ�Ƶõ��ǣ� ��
| A��CuS | B��FeS | C��Al2S3 | D��FeCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й����ʵ����ʺ����ʵ�Ӧ�þ���ȷ����
| A��H2O2��һ����ɫ���������������Ը���������ò��� |
| B��SiO2�������а뵼�����ʣ��������������ά |
| C��ͭ�Ľ�����Ա�����������ͭ������������Ũ���� |
| D�����������õĵ����ԣ��ֿ���ǿ�ȴ�о�����߿�����Զ�����ѹ����߲��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�������Դ����������ȡ�봢����������Դ����������о��ȵ㡣
��֪��CH4(g)��H2O(g)=CO(g)��3H2(g)��H ����206.2 kJ��mol��1
CH4(g)��CO2(g)=2CO(g)��2H2(g)��H ����247.4 kJ��mol��1
2H2S(g)=2H2(g)��S2(g)��H ����169.8 kJ��mol��1
(1)�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)�� H2O(g)��Ӧ����CO2(g)�� H2(g)���Ȼ�ѧ����ʽΪ_________________________
(2)H2S �ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ���� H2S ȼ�գ���Ŀ����________��ȼ�����ɵ� SO2�� H2S ��һ����Ӧ���������ڳ����¾�Ϊ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ��___________________________
(3)H2O ���ȷֽ�Ҳ�ɵõ� H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ����ʾ��ͼ�� A��B ��ʾ������������______________________________________��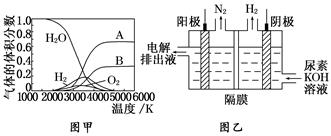
(4)�������[CO(NH2)2]�ļ�����Һ�����װ��ʾ��ͼ��ͼ��(�����и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫)�����ʱ�������ĵ缫��ӦʽΪ_____________________
(5)Mg2Cu ��һ�ִ���Ͻ� 350 ��ʱ��Mg2Cu �� H2��Ӧ������ MgCu2�ͽ���һ�ֽ���Ԫ�ص��⻯��(���������������Ϊ 0.077)��Mg2Cu �� H2��Ӧ�Ļ�ѧ����ʽΪ_____________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС���ú���������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽������ͼ��ʾ��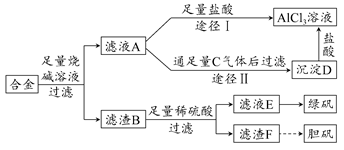
�Իش��������⣺
(1)����ʱ�õ��������У���ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������________��
(2)����ҺA��AlCl3��Һ��;����͢�����������Ϊ��������________��������_____________________________��
(3)����ҺE�еõ��̷������ʵ�������________��
(4)д��������F�Ʊ���������Ļ�ѧ����ʽ_______________________��
(5)��ͬѧ����ɽ���������������ܽ�Ͻ���ռ��Ϊ���ᣬ������Ʒ�����Ҳ���Ƶ����������ʣ�����Ϊ���ߵķ����Ƿ������________��������________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪X��Y��Z��M��Q��G��R��T��ǰ�����ڵ�8��Ԫ�أ�δ����ԭ���������У���ZԪ�ص�ij���⻯������������ȼ�ϣ�QԪ�صĵ���Ϊ��ɫֲ�������õ�һ�ֲ�����ݻ�ѧ�Խ��߷���GԪ�ضԽ��ߵ���һ��Ԫ��T�ĵ��ʿ�����RԪ���������������Ӧˮ��Һ�в���Ŀǰ�����ް���GԪ����ࣻYԪ�صļ۵����Ų�ʽΪ��n+1��Sn ��n+1��P��n+3���� XԪ��ij�ֵ�����Һ������MԪ�صĵ��ʷ�����Ӧ����������XԪ�صĵ��ʣ������ߺ˵�������3����MԪ�صĵ����ǵؿǺ����ڶ��ߵĽ���Ԫ�أ�RԪ��Ϊ�������ڵ�ij��Ԫ�ء�
��1��д��XԪ�صļ۵����Ų�ʽ ��ZԪ�ض�Ӧ������⻯��Ŀռ乹�� ��
��2��д��Ԫ��T�ĵ��ʿ�����RԪ���������������Ӧˮ��Һ��Ӧ�����ӷ���ʽ��
��
��3��QԪ������Ӧ���⻯��ķе��ͬ����Ԫ������Ӧ�⻯��е�ߵ�ԭ���ǣ�
��
��4�������У�Ӧ����Y��QԪ�ص�ij�ַ�ĩ״�����ܷ⡢�ܹ⡢���ﱣ�棬�Ҹ÷�ĩ״���ʾ�����ϴ�·�ʱ�õ������û�ѧ����ʽ˵���������ʳ����ڿ���ʧЧ��ԭ��
��
��5��ZԪ�ص�ij���⻯����������ȼ�ϣ��Ҹû�������Zԭ������ԭ�ӵĸ�����Ϊ1��2����ҵ�������غ�Ư��Һ����Ҫ��Ч�ɷ֣���RԪ���������������Ӧˮ��Һ�в��ø��������Ϊ������Ӧ����������Ϊˮ�ϰ��Ļ�������ɵ�����������ij�����ӣ�������CaCl2��Һ��Ӧ����ij�ְ�ɫ����������ʯ����Ҫ�����ֳɷ֡�ͨ���������ϣ�д���û�ѧ����ʽ��
��
��6����ȡMԪ�ص�ij�����������Թ��У�����ϡ�����pHԼΪ7���������-KI��Һ��QԪ�ص�ij�ֻ�����Ҹû�����Ŀռ乹��Ϊ���������Σ���Ӧ�����Һ����ɫ���к��ɫ�������ɡ�������2mol I-ʱ����ת��3mol���ӣ��÷�Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ij�о���ѧϰС����ƵĶ�һ�ַϾɺϽ���ɷ֣�����Cu��Fe��Si ���ֳɷ֣����з��롢���������õĹ�ҵ���̣�ͨ�������̽����ɷ�ת��Ϊ���õĵ��ʼ������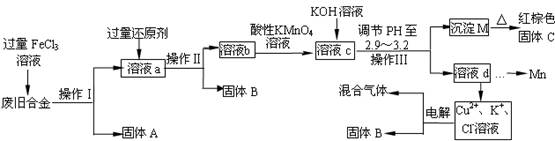
��֪��298Kʱ��Ksp[Cu(OH)2]=2��2��10-20��Ksp[Fe(OH)3]=4��0��10-38��Ksp[Mn(OH)2]=1��9��10-13��
�����������̻ش��й����⣺
��1��������ָ���� ��
��2���������FeCl3��Һ�����п����漰�Ļ�ѧ����ʽ�� ��
��3�������Ļ�ԭ��Ӧ�� ����Һb�������Ľ����������� ��
��4��������Һb�м�������KMnO4��Һ������Ӧ�����ӷ���ʽΪ ��
������Xmol/LKMnO4��Һ������Һb����ǡ�÷�Ӧʱ����KMnO4��ҺYmL����������ú���ɫ����C������Ϊ g���ú�X��Y�Ĵ���ʽ��ʾ����
��5��������,����Һc�������Ľ���������Ũ����ȣ�����Һc����μ���KOH��Һ�������ֽ��������ӳ������Ⱥ�˳��Ϊ�� �� �� ��(�����������)
��6�����һ��������ö��Ե缫���һ��ʱ�����������B������ΪZg��ͬʱ������������ռ��������������ȣ��������������ɵ����һ���������Ϊ L���ú�Z�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����г�ȥ�������õ��Լ��Ͷ�Ӧ�����ӷ���ʽ��
��1��FeCl2��Һ����FeCl3���ʣ������Լ�___________�����ӷ���ʽ_________________________ ��
��2��CO2�������HCl���ʣ������Լ�_____________�����ӷ���ʽ____________________________��
��3��NaHCO3��Һ����Na2CO3���ʣ������Լ�________�����ӷ���ʽ___________________________��
��4��NO�л���NO2���ʣ������Լ�_____________�����ӷ���ʽ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������X���ȷֽ⣬�ɵ�A��B��D��E��F��ˮ���ֲ������A��B��D������ѧ��ѧ�г��������������E�ǵ���F����Ԫ�ص��⻯�
��1��A������ǿ�ᡢǿ�д��A��ǿ����Һ��Ӧ�����ӷ���ʽ_______________��
��2��B��D�������������������Ԫ����ͬ��D����ˮ��ǿ�ᣬ��B��D�����г���Ԫ����������һ��Ԫ�������ڱ��е�λ����_______________��
��3��E��ʹʪ��ĺ�ɫʯ����ֽ������ʵ������ȡE����Ļ�ѧ����ʽΪ________________���Ƶõ����������ͼ��ʾװ���ռ���������Ӧ��_______(�A����B��)ͨ�롣
��4���ɸ��ֽ��������ʵ���֮���Ʋ�X��������������������仯ѧʽΪ___________������X��Ũ��Һ�еμ�ŨNaOH��Һ����������������Ϊ_______________ ��___________ _��
_____________ ��
��5��ȡһ������X����ֽ⣬������F 1 mol�����ͬʱ����_______ ����_____ mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com