
·ÖĪö £Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗµÄŗĖŠÄŅĒĘ÷ŹĒČŻĮæĘ棻
£Ø2£©øł¾Żm=CVMĄ“¼ĘĖć£»
£Ø3£©¶ØČŻŹ±ŅŖÓĆ½ŗĶ·µĪ¹ÜÖšµĪ¼ÓČė£¬ÖĮ°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ£»
£Ø4£©øł¾Żc=$\frac{n}{V}$²¢½įŗĻČÜÖŹµÄĪļÖŹµÄĮænŗĶČÜŅŗµÄĢå»żVµÄ±ä»ÆĄ“½ųŠŠĪó²ī·ÖĪö£»
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗµÄŗĖŠÄŅĒĘ÷ŹĒČŻĮæĘæ£¬ČŻĮæĘæÉĻ±źÓŠČŻ»ż”¢ĪĀ¶ČŗĶĪØŅ»Ņ»ĢõæĢ¶ČĻߣ¬ÓÉÓŚŹµŃéŹŅĪŽ80mLČŻĮæĘ棬¹ŹÓ¦Ń”Ōń100mLµÄČŻĮæĘ棬¹Ź“š°øĪŖ£ŗ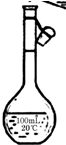 £»
£»
£Ø2£©ÅäÖĘ80mL 0.1mol/LµÄFeSO4ČÜŅŗ£¬ÓÉÓŚ80mLČŻĮæĘ棬¹ŹÓ¦Ń”Ōń100mLµÄČŻĮæĘ棬ÅäÖĆ³ö100mLČÜŅŗ£¬¹ŹĖłŠčµÄFeSO4•7H2OÖŹĮæm=CVM=0.1mol/L”Į0.1L”Į280g/mol=2.8g£¬¹Ź“š°øĪŖ£ŗ2.8g£»
£Ø3£©¶ØČŻµÄ²Ł×÷ĪŖ£ŗµ±ŅŗĆęĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜÖšµĪ¼ÓÕōĮóĖ®ÖĮ°¼ŅŗĆę×īµĶµćÓėæĢ¶ČĻßĻąĒŠ£¬¹Ź“š°øĪŖ£ŗµ±ŅŗĆęĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜÖšµĪ¼ÓÕōĮóĖ®ÖĮ°¼ŅŗĆę×īµĶµćÓėæĢ¶ČĻßĻąĒŠ£»
£Ø4£©¢ŁČōČŻĮæĘæĪ“øÉŌļ¼“ÓĆĄ“ÅäÖĘČÜŅŗ£¬¶ŌČÜŅŗÅضČĪŽÓ°Ļģ£¬ŅņĪŖÖ»ŅŖ¶ØČŻŹ±ÕżČ·£¬ÖĮÓŚĖ®ŹĒŌĄ“¾ĶÓŠµÄ»¹ŹĒŗóĄ“¼ÓČėµÄ£¬¶ŌÅضČĪŽÓ°Ļģ£¬¹Ź“š°øĪŖ£ŗĪŽÓ°Ļģ£»
¢Ś×ŖŅĘČÜŅŗ¹ż³ĢÖŠ³öĻÖĀ©Ņŗ£¬»įµ¼ÖĀČÜÖŹµÄĖšŹ§£¬ŌņÅضČĘ«µĶ£¬¹Ź“š°øĪŖ£ŗĘ«µĶ£»
¢ŪĪ“ÓĆÕōĮóĖ®Ļ“µÓÉÕ±£¬»įµ¼ÖĀČÜÖŹµÄĖšŹ§£¬ŌņÅضČĘ«µĶ£¬¹Ź“š°øĪŖ£ŗĘ«µĶ£»
¢Ü¶ØČŻŹ±ø©ŹÓ£ØŹÓĻßĘ«øߣ©£¬»įµ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ŌņÅضČĘ«øߣ¬¹Ź“š°øĪŖ£ŗĘ«øߣ»
¢ŻŅ”ŌČŗó·¢ĻÖŅŗĆę½µµĶŹĒÕż³£µÄ£¬ŌŁ¼ÓÕōĮóĖ®»įµ¼ÖĀČÜŅŗÅضČĘ«µĶ£¬¹Ź“š°øĪŖ£ŗĘ«µĶ£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ¹ż³ĢÖŠµÄ¼ĘĖćŗĶĪó²ī·ÖĪö£¬ŹōÓŚ»ł“”ŠĶĢāÄ棬ÄŃ¶Č²»“ó£®


 ×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
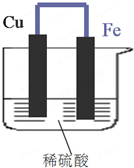 ČēĶ¼ĖłŹ¾£¬½«Ģś”¢ĶĶعżµ¼ĻßĻąĮ¬£¬ÖĆÓŚĻ”ĮņĖįÖŠ£®
ČēĶ¼ĖłŹ¾£¬½«Ģś”¢ĶĶعżµ¼ĻßĻąĮ¬£¬ÖĆÓŚĻ”ĮņĖįÖŠ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
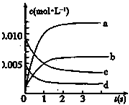 ŌŚ2LĆܱÕČŻĘ÷ÄŚ£¬800”ꏱ·“Ó¦£ŗ2XY £Øg£©+Y2£Øg£©?2XY2£Øg£©ĢåĻµÖŠ£¬n£ØXY£©Ėꏱ¼äµÄ±ä»ÆČē±ķ£ŗ
ŌŚ2LĆܱÕČŻĘ÷ÄŚ£¬800”ꏱ·“Ó¦£ŗ2XY £Øg£©+Y2£Øg£©?2XY2£Øg£©ĢåĻµÖŠ£¬n£ØXY£©Ėꏱ¼äµÄ±ä»ÆČē±ķ£ŗ| Ź±¼ä£Øs£© | 0 | 1 | 2 | 3 | 4 | 5 |
| n£ØXY£©£Ømol£© | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1£ŗ1 | B£® | 2£ŗ3 | C£® | 1£ŗ4 | D£® | 1£ŗ3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ż | B£® | ¢Ś¢Ū¢Ü | C£® | ¢Ł¢Ū¢Ż | D£® | ¢Ł¢Ū¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ±ź×¼×“æöĻĀ£¬16g O2ŗĶO3»ģŗĻĪļÖŠĖłŗ¬Ō×ÓŹżĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬µČĢå»ż Cl2ŗĶCCl4ÖŠĖłŗ¬ĀČŌ×ÓŹżĪŖ1©s2 | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬14g N2ŗ¬ÓŠµē×ÓŹżĪŖ7NA | |
| D£® | 28g COÓė22.4L CO2Ėłŗ¬µÄĢ¼Ō×ÓŹż¾łĪŖNA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŌ¹Ü | B£® | ÉÕĘæ | C£® | ĮæĶ² | D£® | Õō·¢Ćó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀ³£Ń¹ĻĀ£¬11.2 LĀČĘųĖłŗ¬ÓŠµÄŌ×ÓŹżÄæĪŖNA | |
| B£® | 9 gĖ®Ėłŗ¬ÓŠµÄĒāŌ×ÓŹżÄæĪŖNA | |
| C£® | ŌŚĶ¬ĪĀĶ¬Ń¹Ź±£¬ĻąĶ¬ĪļÖŹµÄĮæµÄČĪŗĪĘųĢåµÄĢå»żĻąĶ¬ĒŅĪŖ11.2L | |
| D£® | 0.1 mol¼×Ķé·Ö×Óŗ¬ÓŠµÄŌ×ÓŹżÄæĪŖNA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com