
(14��)��1����֪:
Fe��s��+1/2O2��g��=FeO��s�� 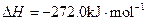
2Al��s��+3/2O2��g��= Al2O3��s�� 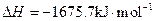
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��________________________________________��
��2����Ӧ�����������Ϊ��̬��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B����ͼ��ʾ��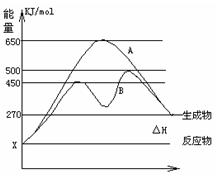
�پ�ͼ�жϸ÷�Ӧ��_____(������š�) �ȷ�Ӧ������Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����__ _ _ (���������С�����䡱)
������B���̱����˷�Ӧ���õ�����Ϊ______ (ѡ�����������ĸ)
A�������¶� B������Ӧ���Ũ��
C�������¶� D��ʹ���˴���
��3�� 1000��ʱ���������������������з�Ӧ��Na2SO4(s) + 4H2(g) 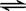 Na2S(s) + 4H2O(g) ��
Na2S(s) + 4H2O(g) ��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ____________________����֪K1000����K1200������÷�Ӧ��________��Ӧ������ȡ����ȡ�����
�����й����ӷ���ʽ˵��������Ӧ���ù�������ˮ��Һ�������_______ _____
��4�������£����ȡ0.1mol��L��1HA��Һ��0.1mol��L��1NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH��8����ش��������⣺
�ٻ����Һ��ˮ�������c(H��)��0.1mol��L��1NaOH��Һ��ˮ�������c(H��)�Ƚ�
���������������
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH 7�����������������ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ ��������ţ�
a.NH4HCO3 b.NH4A c.(NH4)2CO3 d.NH4Cl
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�����ظ�����ѧ�߶��ڶ����¿���ѧ�Ծ����������� ���ͣ������
(14��)ȡ0.1 mol/L HA��Һ��0.1 mol/L NaOH��Һ��������(��Ϻ���Һ����ı仯����)����û����Һ��pH��8���Իش��������⣺
(1)�����Һ��pH��8��ԭ����________________________________(�����ӷ���ʽ��ʾ)��
(2)�����Һ����ˮ�������c(H��)________0.1 mol/L NaOH��Һ����ˮ�������c(H��) (�>������<������)��
(3)������Һ��������ʽ�ľ�ȷ������(���������)��
c(Na��)��c(A��)��________mol/L��c(OH��)��c(HA)��________mol/L��
(4)��֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(�>������<������)��
(5)����ͬ�¶�����ͬŨ�ȵ���������Һ��A.NH4HCO3��B.NH4A��C.(NH4)2SO4��D.NH4Cl����pH�ɴ�С��˳������________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��������ˮһ�и�����ѧ����ĩģ�⻯ѧ�Ծ����������� ���ͣ������
(14��)
��1�������±��и�����Ų����ɣ����˹����Ų���22��ӦΪ ��
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| C2H4 | C2H6 | C2H6O | C2H4O2 | C3H6 | C3H8 | C3H8O | C3H6O2 | C4H8 | C4H10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����ѧ����ĩģ�⻯ѧ�Ծ��������棩 ���ͣ������
(14��)
��1�������±��и�����Ų����ɣ����˹����Ų���22��ӦΪ ��
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
C2H4 |
C2H6 |
C2H6O |
C2H4O2 |
C3H6 |
C3H8 |
C3H8O |
C3H6O2 |
C4H8 |
C4H10 |
��2�����з�Ӧ������ȡ����Ӧ���� ��������ţ�
����ϩʹ���Ը��������Һ��ɫ �ڼ����������Ϲ��� �۱�ʹ��ˮ����ɫ
��������Ҵ���Ӧ ���Ҵ������ȩ �������Ƿ���������Ӧ
��A��B��C��D��E��FΪԭ������������������ֶ�����Ԫ�ء������£�����Ԫ�صij�������������Ϊ���壬����Ϊ���塣A��E��D��F�ֱ�ͬ���壬A����B��C��D�ֱ��γɵ�������ȵ����ַ��ӣ�C��D������������֮����E�ĺ������������ȡ��Իش��������⣺
��1��д������Ԫ�ص�Ԫ�ط��ţ� C ��F ��
��2������ʽΪA2B2D4�Ļ������뺬�����ʵ�����KOH����Һ��Ӧ��������Һ�����ԣ���ԭ���� �����÷���ʽ����Ҫ������˵����������Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3����A��C��D��F��Fe����Ԫ�ؿ�������������Ļ�����Z����Է�������Ϊ392����1molZ�к���6mol�ᾧˮ���Ի�����Z��������ʵ�飺
a��ȡZ����Һ�����������NaOHŨ��Һ�����ȣ�������ɫ��������ɫ�д̼�����ζ�����塣��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
b����ȡZ����Һ�����������BaCl2��Һ������ɫ������������������ܽ⡣
��Z�Ļ�ѧʽΪ ��
����֪100mL1mol/LZ��Һ����20mL1mol/LKMnO4��Һ�������ữ��ǡ�÷�Ӧ��д���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꺣�������λ���ѧ������ѧ�ڸ��н�ѧ������⣨������ѧ�Ծ��������棩 ���ͣ������
(12��)
��1����֪H2A��ˮ�д������µ��룺H2A H����HA����HA��
H����HA����HA�� H����A2�����ش����⣺
H����A2�����ش����⣺
��NaHA��Һ�� (����ԡ��������ԡ��������ԡ�����ȷ����)��
��ij�¶��£���0.1mol��L-1��NaHA��Һ�е���0.1mol��L-1KOH��Һ�����ԣ���ʱ��Һ�����¹�ϵһ����ȷ���� (����ĸ)��
A��c(H+)�� c(OH-) =1��10-14
B��c(Na+)+c(K+)=2c(A2-)+c(HA-)
C��c(Na+)=0.05mol ��L-1
D��c(Na+)= c(A2-)+c(HA-) +c(H2A)
��2����֪�����£�CaA(s) Ca2+(aq)��A2-(aq)����H>0��c(Ca2��)��c(A2��)Ϊ������������
Ca2+(aq)��A2-(aq)����H>0��c(Ca2��)��c(A2��)������������
Ksp��c(Ca2��)��c(A2��)���Իش�
���¶�����ʱ��Ksp (���������С�����䡱����ͬ)
�ڵμ�����Ũ���ᣬc(Ca2+) ��ԭ����
��3��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8��10��9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2��10��4mo1/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и�����һ���¿���ѧ�Ծ��������棩 ���ͣ������
�����14�֣�5������ͪ��������һ�ֿ�����ҩ����ϳ�·�����£�
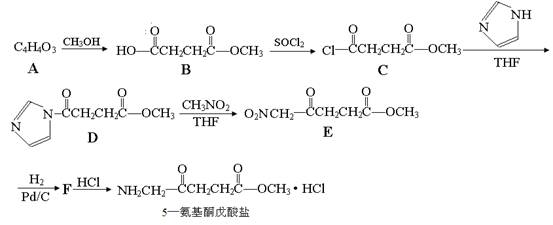
��֪��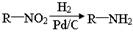
��1����֪A���ӽṹ����һ������A����ʹ��ˮ��ɫ���Һ˴Ź�������ͼ��ֻ��һ���壬��A�Ľṹ��ʽΪ ��
��2��5������ͪ�������к��������ŵ������� ��C��D�ķ�Ӧ����Ϊ ��
��3��G��B��һ��ͬ���칹�壬����NaHCO3��Һ��Ӧ���ܷ���������Ӧ��1molG����������Na��Ӧ������1molH2����G�����в�������д��һ�ַ�������������G�Ľṹ��ʽ ��
��4��д��D��E�ķ�Ӧ����ʽ ��
��5����֪ ����������������Ϣ��д����CH3CH2COOH��
����������������Ϣ��д����CH3CH2COOH�� Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� ����ĺϳ�·������ͼ�����Լ���ѡ����
����ĺϳ�·������ͼ�����Լ���ѡ����
�ϳ�·������ͼʾ�����£�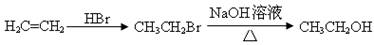
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com