
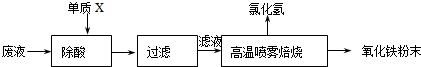
��������ҵ��ϴ�ֲ�ʱ�����ķ�Һ��Ҫ�ɷ�ΪFe2+��H+��Cl���������������������÷�Һ���������ᣬ�Ʊ�������Ϳ�ϡ�
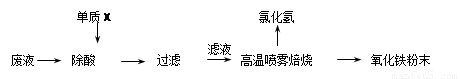
��1������X�Ļ�ѧʽ�� ��
��2���Ȼ�������Һ��������������ʱת��Ϊ�Ȼ����������������ĩ���йصĻ�ѧ����ʽΪ��
��3��ij������Ϳ�ϵijɷ��У�������Fe2O3�⣬��������CuO��FeO�е�һ�֡������ʵ��̽��������Ϳ�������ӵ����ʡ�
�������������
����1��������CuO�� ����2��������FeO��
�ڻ��ڼ��� ���1����2���������ʵ�鷽��������ʵ�顣�ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡʵ���Լ������ۡ�3mol•L��1H2SO4��0.01 mol•L��1����KMnO4��Һ��10%NaOH��Һ��10%H2O2��KSCN��Һ
|
�������� |
Ԥ������ͽ��� |
|
����1��ȡ������Ʒ���Թ��У�_________________ ____________________________________________ |
___________________________ |
|
����2�� ____________________________________________ |
___________________________ |
��4����ˮ���ա������������ա�ʱ�������Ȼ�������ɵõ����ᡣ����㣺��1000gˮ�����ձ�״���¶������Ȼ�������ɵõ�36.5%��Ũ�����д��������̣����������ѧ����ʽ��ʾ�����ػ���
��16�֣�
��1��Fe ��1�֣�
��2��FeCl2 + 2H2O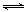 Fe(OH)2 +
2HCl
��1�֣�
Fe(OH)2 +
2HCl
��1�֣�
4Fe(OH)2 + O2 +2H2O = 4 Fe(OH)3 ��1�֣�
2Fe(OH)3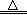 Fe2O3 + 3H2O
��1�֣�©д�������÷֣�
Fe2O3 + 3H2O
��1�֣�©д�������÷֣�
����4FeCl2+4H2O+O2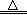 2 Fe2O3+8HCl
��3�֣�©д������2�֣�
2 Fe2O3+8HCl
��3�֣�©д������2�֣�
��3������1 ��1�֣�
������ÿ��2�֣���8�֣�
|
ʵ����� |
Ԥ������ͽ��� |
|
����1������������3mol•L��1H2SO4������� |
��Ʒȫ���ܽ� |
|
����2��ȡ������Һ���Թ��У������������ۣ�������ټ�������3mol•L��1H2SO4������� |
�Թ��г��ֺ�ɫ���壬˵����������CuO |
������˵��������1��δ�ӡ��������͡��������1�֡�
����2�з���Ϊ��������10%NaOH��Һ�����������ɫ������˵����������CuO��������2�ͽ��۵�0�֣���Ϊ��Fe3+��H+���ţ���һ���ܹ۲쵽��ɫ������
����2 ��1�֣�
|
ʵ����� |
Ԥ������ͽ��� |
|
����1������������3mol•L��1H2SO4������� |
��Ʒȫ���ܽ� |
|
����2��ȡ������Һ���Թ��У���μ���0.01 mol•L��1����KMnO4��Һ |
����Ϻ�ɫ��ȥ��˵����������FeO��
|
������˵��������1��δ�ӡ��������͡��������1�֡�
����2�У���KSCN��H2O2����ϼ���Fe2+������2�ͽ��۵�0�֣���Ϊ�� Fe3+���š�
��4����3�֣��������HCl���ΪxL����
(36.5x/22.4)/(1000+ 36.5x/22.4) ��100%=36.5% ��2�֡���ȷ��ʽ��2�֣�
���x= 22400/63.5(L)=352.89(L) ��1�֣�������ȷ�Ĵ�Ҳ�÷֣�
��������
�����������1�����ݳ�ȥ�����ʲ�����������ԭ��Fe+2H+=Fe2++H2������˳��Ჽ��ѡ��ĵ���Xһ���ǹ��������ۻ���м����2���Ȼ�������ǿ�������Σ���ˮ�⣬����ˮ�ⷴӦ�����ȷ�Ӧ����˸�����ʹ�䳹��ˮ�⣬�����������������Ȼ��⣻�Ȼ��������ݳ����������������ȶ������ױ������е���������Ϊ����������������������ʱ�ֽ⣬������������ˮ����������������Ӧ���Եõ��ܷ�Ӧʽ����3��������1������Fe2O3��CuO����������ˮ�ļ������������ֱ��ѡ���Լ�����CuO�Ĵ��ڣ�������������ϡ�����ܽ����������������ͭ��Һ���ٸ��ݽ������˳��������Ρ�ͭ�ε����ʣ�Fe+Fe2(SO4)3=3FeSO4��Fe+CuSO4=FeSO4+Cu����˼�����������ۣ���ַ�Ӧ��������ɫ���壬˵����Ʒ�к���CuO����ѡ��NaOH��Һ��۲���Һ����ɫ�����У���Ϊ�����Ӷ�ͭ���ӵļ�����ɸ��ţ�������2������Fe2O3��FeO����������ˮ�ļ������������ֱ��ѡ���Լ�����FeO�Ĵ��ڣ�������������ϡ�����ܽ����������������������Һ���ٸ����Ρ������ε����ʣ�������Һ����ʹ���Ը��������Һ��ɫ������������Һ���ܣ���ӦΪ5Fe2++MnO4��+8H+=5Fe3++Mn2++4H2O����˼����������Ը��������Һ����ַ�Ӧ����Һ��ɫ��˵����Ʒ�к���FeO������ѡ��KSCN��˫��ˮ���飬��Ϊ�����ӻ�����������ӵļ��飻��4���������HCl���ΪxL������n=V/22.4L•mol��1��m=n•M��m(��Һ)=m(����)+m(ˮ)��w(����)=m(����)/m(��Һ)��100%����(36.5x/22.4)/(1000+ 36.5x/22.4) ��100%=36.5% �����x= 22400/63.5(L)=352.8(L)��
���㣺���黯ѧ�������̴��⡢̽��ʵ������ƴ�̣��漰����Ԫ�ؼ��仯�������Ҫ���ʡ�����ˮ�⡢����Ħ����������ʵ��������������������ļ���ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| �������� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ���Թ��У� ����������3mol?L-1H2SO4������� ����������3mol?L-1H2SO4������� |
��Ʒȫ���ܽ� ��Ʒȫ���ܽ� |
| ����2�� ȡ������Һ���Թ��У������������ۣ�������ټ�������3mol?L-1H2SO4������� ȡ������Һ���Թ��У������������ۣ�������ټ�������3mol?L-1H2SO4������� |
�Թ��г��ֺ�ɫ���壬˵����������CuO �Թ��г��ֺ�ɫ���壬˵����������CuO |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 22400 |
| 63.5 |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 36.5V |
| 22.4 |
| 22400 |
| 63.5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com