
ÓŠX”¢Y”¢ZČżÖÖŌŖĖŲ£¬ŅŃÖŖ£ŗ¢ŁX”¢Y”¢ZµÄµ„ÖŹŌŚ³£ĪĀĻĀ¾łĪŖĘųĢ壻¢ŚXµ„ÖŹæÉŌŚZµ„ÖŹÖŠČ¼ÉÕ£¬Éś³ÉĘųĢ¬XZ£»¢ŪXZ¼«Ņ×ČÜÓŚĖ®£¬µēĄė³öX+ŗĶZ££¬ĘäĖ®ČÜŅŗæÉŹ¹Ą¶É«ŹÆČļŹŌÖ½±äŗģ£»¢ÜĆæ2øöX2·Ö×ÓæÉÓė1øöY2·Ö×Ó»ÆŗĻÉś³É2øöX2Y·Ö×Ó£¬X2YŌŚ³£ĪĀĻĀĪŖĪŽÉ«ŅŗĢ壻¢ŻZµ„ÖŹČÜÓŚX2YÖŠ£¬ĖłµĆČÜŅŗ¾ßÓŠĘÆ°××÷ÓĆ”£
£Ø1£©ĶʶĻX£¬YĮ½ÖÖŌŖĖŲ£ŗX________£¬Y_________£ØĢīŌŖĖŲĆū³Ę£©”£
£Ø2£©Š“³ö¢Ż¹ż³ĢÖŠµÄ»Æѧ·“Ó¦µÄ·½³ĢŹ½ ”£
£Ø3£©Š“³ö¹¤ŅµÉĻÖĘČ”Zµ„ÖŹµÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©Š“Zµ„ÖŹÓėŹÆ»ŅČé·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø1£©Ēā Ńõ £Ø2£©Cl+H2O HCl+HClO
HCl+HClO
£Ø3£©2NaCl£«2H2O 2NaOH£«H2”ü£«Cl2”ü £Ø4£©2Ca(OH)2 +2Cl2£½Ca(ClO)2+CaCl2+2H2O
2NaOH£«H2”ü£«Cl2”ü £Ø4£©2Ca(OH)2 +2Cl2£½Ca(ClO)2+CaCl2+2H2O
½āĪöŹŌĢā·ÖĪö£ŗXµÄµ„ÖŹŌŚZµÄµ„ÖŹÖŠČ¼ÉÕ£¬Éś³ÉXZ£¬²¢ĒŅXZ¼«Ņ×ČÜÓŚĖ®£¬ŌŚĖ®ČÜŅŗÖŠµēĄė³öX+ŗĶZ-”£XZµÄĖ®ČÜŅŗæÉŹ¹ŹÆČļŹŌŅŗ±äŗģ£¬ĖµĆ÷XĪŖH2£¬ZĪŖCl2£¬XZĪŖHCl£»Į½·Ö×ÓXµÄµ„ÖŹæÉÓėŅ»·Ö×ÓYµÄµ„ÖŹ»ÆŗĻÉś³ÉĮ½·Ö×ÓX2Y£¬X2Y³£ĪĀĻĀĪŖŅŗĢ壬ĖµĆ÷YĪŖO2£¬X2YĪŖH2O”£Cl2ČÜÓŚH2OÖŠ£¬·“Ӧɜ³ÉHClŗĶHClO£¬HClO¾ßÓŠĘÆ°××÷ÓĆ”£
£Ø1£©ĶعżŅŌÉĻ·ÖĪöÖŖ£¬XŗĶYŌŖĖŲĆū³Ę·Ö±šŹĒĒā”¢Ńõ£¬¹Ź“š°øĪŖ£ŗĒā£»Ńõ£»
£Ø2£©Cl2ČÜÓŚH2OÖŠ£¬·“Ӧɜ³ÉHClŗĶHClO£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCl+H2O HCl+HClO”£
HCl+HClOӣ
£Ø3£©¹¤ŅµÉĻÓƵē½ā±„ŗĶĀČ»ÆÄĘČÜŅŗµÄ·½·ØÖĘČ”ĀČĘų£¬·“Ó¦·½³ĢŹ½ĪŖ2NaCl£«2H2O 2NaOH£«H2”ü£«Cl2”ü”£
2NaOH£«H2”ü£«Cl2”ü”£
£Ø4£©ĀČĘųŗĶĒāŃõ»ÆøĘ·“Ӧɜ³ÉĀČ»ÆøĘ”¢“ĪĀČĖįøĘŗĶĖ®£¬·“Ó¦·½³ĢŹ½ĪŖ2Ca(OH)2£«2Cl2£½Ca(ClO)2£«CaCl2£«2H2O
æ¼µć£ŗæ¼²éĪļÖŹĶʶĻ”¢ŅŌ¼°ĀČĘų¹¤ŅµÖʱøŗĶŠŌÖŹ


 דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø
דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
NOxŹĒĘū³µĪ²ĘųÖŠµÄÖ÷ŅŖĪŪČ¾ĪļÖ®Ņ»”£
(1)NOxÄÜŠĪ³ÉĖįÓź£¬Š“³öNO2×Ŗ»ÆĪŖHNO3µÄ»Æѧ·½³ĢŹ½£ŗ_________________________________________________________”£
(2)Ęū³µ·¢¶Æ»ś¹¤×÷Ź±»įŅż·¢N2ŗĶO2·“Ó¦£¬ĘäÄÜĮæ±ä»ÆŹ¾ŅāĶ¼ČēĻĀ£ŗ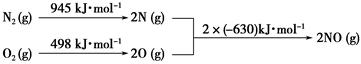
¢ŁŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ_______________________________________”£
¢ŚĖęĪĀ¶ČÉżøߣ¬øĆ·“Ó¦»ÆŃ§Ę½ŗā³£ŹżµÄ±ä»ÆĒ÷ŹĘŹĒ____”£
(3)ŌŚĘū³µĪ²ĘųĻµĶ³ÖŠ×°ÖĆ“ß»Æ×Ŗ»ÆĘ÷£¬æÉÓŠŠ§½µµĶNOxµÄÅÅ·Å”£
¢Łµ±Ī²ĘųÖŠæÕĘų²»×揱£¬NOxŌŚ“ß»Æ×Ŗ»ÆĘ÷ÖŠ±»»¹Ō³ÉN2Åųö”£Š“³öNO±»CO»¹ŌµÄ»Æѧ·½³ĢŹ½£ŗ____________________________________”£
¢Śµ±Ī²ĘųÖŠæÕĘų¹żĮæŹ±£¬“ß»Æ×Ŗ»ÆĘ÷ÖŠµÄ½šŹōŃõ»ÆĪļĪüŹÕNOxÉś³ÉŃĪ”£ĘäĪüŹÕÄÜĮ¦Ė³ŠņČēĻĀ£ŗ12MgO£¼20CaO£¼38SrO£¼56BaO”£ŌŅņŹĒ_____________________________________________£¬
ŌŖĖŲµÄ½šŹōŠŌÖš½„ŌöĒ棬½šŹōŃõ»ÆĪļ¶ŌNOxµÄĪüŹÕÄÜĮ¦Öš½„ŌöĒ攣
(4)ĶعżNOx“«øŠĘ÷æɼą²āNOxµÄŗ¬Į棬Ę乤×÷ŌĄķŹ¾ŅāĶ¼ČēĻĀ£ŗ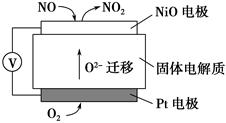
¢ŁPtµē¼«ÉĻ·¢ÉśµÄŹĒ________·“Ó¦(Ģī”°Ńõ»Æ”±»ņ”°»¹Ō”±)
¢ŚŠ“³öNiOµē¼«µÄµē¼«·“Ó¦Ź½£ŗ_______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŌĻĀŹĒ¶ŌÖŲŅŖ·Ē½šŹō¼°Ęä»ÆŗĻĪļµÄĢÖĀŪ£¬øł¾ŻŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©ŹµŃéŹŅŹ¢×°NaOHČÜŅŗŹĒŹŌ¼ĮĘæ²»ÄÜÓĆ²£Į§Čū£¬Ó¦øĆÓĆĻš½ŗČū£¬ŅŌ·ĄÖ¹·¢Éś·“Ó¦£ŗ
£ØĄė×Ó·½³ĢŹ½£©”£
£Ø2£©°±ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤²śĘ·£¬ĆܶȱČæÕĘų £ØĢī”°“ó”±»ņ”°Š””±£©”£¹¤ŅµÉĻÖʱø°±ĘųµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©¹¤ŅµÉĻÖĘČ”ĘÆ°×·ŪµÄ·“Ó¦»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ÅØH2SO4³£ÓĆ×÷ĘųĢåøÉŌļ¼Į£¬ŹĒŅņĪŖĖü¾ßÓŠ____________£»½«ŹŹĮæµÄÕįĢĒ·ÅČėÉÕ±ÖŠ£¬µĪČė¼øµĪĖ®£¬½Į°č¾łŌČ”£Č»ŗó¼ÓČėŹŹĮæÅØĮņĖį£¬ŃøĖŁ½Į°č£¬·Å³ö“óĮæµÄČČ£¬Ķ¬Ź±¹Ū²ģµ½ÕįĢĒÖš½„±äŗŚ£¬Ģå»żÅņÕĶ£¬²¢·Å³öÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ唣Ēė»Ų“š£ŗ
²śÉś“Ģ¼¤ŠŌĘųĪ¶ĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©ĶŗĶĻ”ĻõĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£Čō²Ī¼Ó·“Ó¦µÄCuÖŹĮæĪŖ6.4g£¬Éś³ÉNOĘųĢå____________L£Ø±ź×¼×“æöĻĀ£©£¬Ōņ×ŖŅʵē×ÓĪļÖŹµÄĮæĪŖ mol£¬±»»¹ŌµÄÓėĪ“±»»¹ŌµÄHNO3ĪļÖŹµÄĮæÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øß“æ¹čÉś²śĮ÷³ĢČēĻĀ£ŗ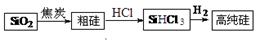
£Ø1£©ÓÉSiO2ÖĘ“Ö¹čµÄ»Æѧ·½³ĢŹ½ŹĒ £¬øĆ·“Ó¦²»ÄÜĖµĆ÷Ģ¼µÄ·Ē½šŹōŠŌĒæÓŚ¹č£¬ŌŅņŹĒ £¬ĒėŠ“³öŅ»øöÄÜĖµĆ÷Ģ¼µÄ·Ē½šŹōŠŌĒæÓŚ¹čµÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©900”ęŅŌÉĻ£¬H2ÓėSiHCl3·¢Éś·“Ó¦£ŗSiHCl3(g)+ H2(g) Si(s) + 3HCl(g) ¦¤H£¾0”£½«Ņ»¶ØĮæµÄ·“Ó¦ĪļĶØČė¹Ģ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
Si(s) + 3HCl(g) ¦¤H£¾0”£½«Ņ»¶ØĮæµÄ·“Ó¦ĪļĶØČė¹Ģ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
a£®ŌŚŗćĪĀĢõ¼žĻĀ£¬ČōČŻĘ÷ÄŚŃ¹Ēæ²»±ä£¬ŌņøĆ·“Ó¦Ņ»¶Ø“ļµ½»ÆŃ§Ę½ŗāדĢ¬
b£®Ōö“óSiHCl3µÄÓĆĮ棬æÉĢįøßSiHCl3µÄĘ½ŗā×Ŗ»ÆĀŹ
c£®ÉżøßĪĀ¶ČæɼÓæģ·“Ó¦ĖŁĀŹ£¬ĒŅĢįøß¹čµÄ²śĀŹ
£Ø3£©øĆĮ÷³ĢÖŠæÉŅŌŃ»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½šŹōĀĮŌŚĖįŠŌ»ņ¼īŠŌČÜŅŗÖŠ¾łæÉÓėNO3£·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ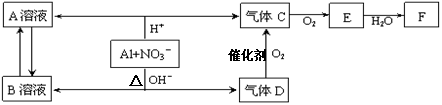
ŅŃÖŖ£¬ĘųĢåDŗĶF·“Ó¦æÉÉś³ÉŃĪ£¬ĘųĢåDŗĶAČÜŅŗ·“Ӧɜ³É°×É«³Įµķ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1) AŗĶBĮ½ČÜŅŗ»ģŗĻ²śÉś°×É«³Įµķ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
(2) C”¢EÅÅČė“óĘųÖŠ»įŌģ³É“óĘųĪŪČ¾£¬ŌŚ“߻ƼĮ“ęŌŚĻĀ£¬DæÉŅŌ½«C»ņE¶¼×Ŗ»ÆĪŖĪŽ¶¾µÄĘųĢ¬µ„ÖŹ£¬ČĪŅāŠ“³öĘäÖŠŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
(3)Š“³öĀĮŌŚ¼īŠŌĢõ¼žĻĀÓėNO3-·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
(4)³żČ„ĘųĢåCÖŠµÄŌÓÖŹĘųĢåEµÄ»Æѧ·½·Ø£ŗ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)
(5)AlÓėNO3£ŌŚĖįŠŌĢõ¼žĻĀ·“Ó¦£¬AlÓė±»»¹ŌµÄNO3£µÄĪļÖŹµÄĮæÖ®±ČŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒø÷ĪļÖŹµÄ·“Ó¦¹ŲĻµĶ¼£ŗŅŃÖŖAŗĶE¶¼ŹĒ»ĘÉ«·ŪÄ©”£FÓŠ“Ģ¼¤ŠŌĘųĪ¶ĒŅÓŠĘư׊Ō³£±»²»·ØÉĢČĖÓĆĄ“ĘÆ°×øÆÖńµČ”£Ēė¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ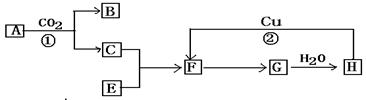
£Ø1£©Š“³öĻĀĮŠø÷ĪļÖŹµÄ»ÆѧŹ½£ŗA£®__________ H£®___________£»
£Ø2£©Š“³ö·“Ó¦¢ŁŗĶ·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł___________________________________________________£»
¢Ś__________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠĮ½ÖÖĪ»ÓŚ¶ĢÖÜĘŚµÄĻąĮŚÖÜĘŚ”¢ĻąĮŚÖ÷×åµÄ·Ē½šŹōŌŖĖŲX”¢Y£¬ŅŃÖŖĮ½ŌŖĖŲ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ¾łĪŖĒæĖį”£øł¾ŻĻĀĶ¼×Ŗ»Æ¹ŲĻµ(·“Ó¦Ģõ¼ž¼°²æ·Ö²śĪļŅŃĀŌČ„)£¬»Ų“šĻĀĮŠĪŹĢā£ŗ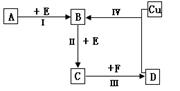
(1)ČōA”¢B”¢C”¢D¾łĪŖŗ¬XŌŖĖŲµÄ»ÆŗĻĪļ£¬ĒŅA”¢FµÄŅ»øö·Ö×ÓÖŠ¶¼Ö»ŗ¬ÓŠ10øöµē×Ó£¬Ōņ£ŗ
¢ŁA·Ö×ÓµÄæռ乹ŠĶĪŖ £¬F·Ö×ӵĵē×ÓŹ½ĪŖ ”£
¢Ś·“Ó¦ I µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢Ū»ÆŗĻĪļNaX3ŹĒŗĻ³É”°“ļ·Ę”±µÄÖŠ¼ä»īŠŌĪļÖŹ£¬Ņ²ŹĒĘū³µ°²Č«ĘųÄŅÖŠµÄÖ÷ŅŖĪļÖŹ”£NaX3ŹÜײ»÷ŗóÉś³ÉNa3XŗĶĮķŅ»ÖÖĘųĢåµ„ÖŹ£¬ĒėŠ“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
¢Ü XµÄŃõ»ÆĪļŹĒŠĪ³É¹ā»ÆѧŃĢĪķŌŅņÖ®Ņ»£¬¹¤ŅµÉĻæɲÉÓĆĖüÓėĘäĒā»ÆĪļ·“Ӧɜ³ÉĪŽ¶¾ŗ¦ĪļÖŹ¶ų³żČ„£¬ĒėÓĆ·½³ĢŹ½±ķŹ¾øĆ·“Ó¦ ”£
(2)ČōA”¢B”¢C”¢D¾łĪŖŗ¬YŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠAÓÉĮ½ÖÖŌŖĖŲ×é³É£¬ĒŅAµÄĦ¶ūÖŹĮæĪŖ120g”¤molØC1£¬Ōņ£ŗ
¢Ł½«·“Ó¦IVĖłµĆµÄČÜŅŗ¼ÓČČÕōøɵƵ½µÄ¾§ĢåŹōÓŚ ¾§Ģå(Ģī”°Ąė×Ó”±”¢”°·Ö×Ó”±”¢”°Ō×Ó”±)
¢Ś·“Ó¦ I µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢Ūŗ¬YŌŖĖŲµÄ»ÆŗĻĪļNa2YŗĶ“ĪĀČĖįÄĘČÜŅŗŌŚĒæ¼īŠŌ»·¾³ÖŠÄÜ·¢Éś·“Ó¦£¬²śĪļĪŽ³Įµķ£¬ĒėŠ“³öøĆ·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
(20·Ö)ijŠ£»ÆѧŹµŃéŠĖȤŠ”×éĪŖĮĖĢ½¾æŌŚŹµŃéŹŅÖʱøCl2µÄ¹ż³ĢÖŠÓŠĖ®ÕōĘųŗĶHCl»Ó·¢³öĄ“£¬Ķ¬Ź±Ö¤Ć÷Cl2µÄijŠ©ŠŌÖŹ£¬¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£ØÖ§³ÅÓƵÄĢś¼ÜĢØŹ”ĀŌ£©£¬°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©ĻĀĮŠ·½·ØÖŠ£¬æÉÖʵƵÄÕżČ·×éŗĻŹĒ__________”£
¢ŁMnO2ŗĶÅØŃĪĖį»ģŗĻ¹²ČČ£» ¢ŚMnO2”¢NaClŗĶÅØĮņĖį»ģŗĻ¹²ČČ£ŗ
¢ŪNaClOŗĶÅØŃĪĖį»ģŗĻ£» ¢ÜK2Cr2O7ŗĶÅØŃĪĖį»ģŗĻ£ŗ
¢ŻKClO3ŗĶÅØŃĪĖį»ģŗĻ¹²ČČ£» ¢ŽKMnO4ŗĶÅØŃĪĖį»ģŗĻ”£
A£®¢Ł¢Ś¢ŽB£®¢Ś¢Ü¢ŽC£®¢Ł¢Ü¢Ž D£®Č«²ææÉŅŌ
£Ø2£©Š“³öŹµŃéŹŅÖĘČ”Cl2µÄĄė×Ó·½³ĢŹ½____________”£
£Ø3£©ČōÓĆŗ¬ÓŠ0.2 mol HClµÄÅØŃĪĖįÓė×ćĮæµÄMnO2·“Ó¦ÖʵĆCl2µÄĢå»ż£Ø±źæöĻĀ£©×ÜŹĒŠ”ÓŚ1.12LµÄŌŅņŹĒ_________________________________________”£
£Ø4£©¢Ł×°ÖĆBµÄ×÷ÓĆŹĒ__________________________________”£
¢Ś×°ÖĆCŗĶD³öĻֵIJ»Ķ¬ĻÖĻóĖµĆ÷µÄĪŹĢāŹĒ________________________”£
¢Ū×°ÖĆEµÄ×÷ÓĆŹĒ_____________________”£
£Ø5£©ŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄŹµŃéӊȱĻŻ£¬²»ÄÜČ·Ļń×īÖÕĶØČėAgNO3ČÜŅŗÖŠµÄĘųĢåÖ»ÓŠŅ»ÖÖ”£ĪŖĮĖČ·±£ŹµŃé½įĀŪµÄæÉææŠŌ£¬Ö¤Ć÷×īÖÕĶØČėAgNO3ČÜŅŗÖŠµÄĘųĢåÖ»ÓŠŅ»ÖÖ£¬ŅŅĶ¬Ń§Ģį³öÓ¦øĆŌŚ×°ÖĆ__________Óė________Ö®¼ä£ØĢī×°ÖĆ×ÖÄøŠņŗÅ£©Ōö¼ÓŅ»øö×°ÖĆ£¬Ōö¼Ó×°ÖĆĄļĆęµÄŹŌ¼ĮæÉĪŖ____________”£
A£®ŹŖČóµÄµķ·ŪKIŹŌÖ½B£®ĒāŃõ»ÆÄĘČÜŅŗ
C£®ŹŖČóµÄŗģÉ«²¼Ģõ D£®±„ŗĶµÄŹ³ŃĪĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖŃŠ¾æĶÓėÅØĮņĖįµÄ·“Ó¦£¬Ä³»ÆѧŠĖȤŠ”×é½ųŠŠČēĻĀŹµŃ锣
ŹµŃé¢ń£ŗ·“Ó¦²śĪļµÄ¶ØŠŌĢ½¾æ”£
ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ£Ø¹Ģ¶Ø×°ÖĆŅŃĀŌČ„£©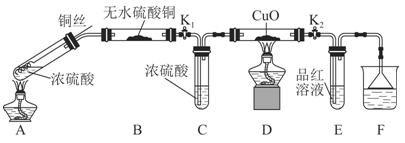
£Ø1£©AÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©FÉÕ±ÖŠµÄČÜŅŗĶس£ŹĒ ”£
£Ø3£©ŹµŃé¹ż³ĢÖŠ£¬ÄÜÖ¤Ć÷ÅØĮņĖįÖŠĮņŌŖĖŲµÄŃõ»ÆŠŌĒæÓŚĒāŌŖĖŲµÄĻÖĻóŹĒ
ӣ
£Ø4£©ŹµŃé½įŹųŗó£¬Ö¤Ć÷A×°ÖĆŹŌ¹ÜÖŠ·“Ó¦ĖłµĆ²śĪļŹĒ·ńŗ¬ÓŠĶĄė×ӵIJŁ×÷·½·ØŹĒ ”£
£Ø5£©ĪŖĖµĆ÷ÅØĮņĖįÖŠµÄĖ®ŹĒ·ńÓ°ĻģB×°ÖĆĻÖĻóµÄÅŠ¶Ļ£¬»¹Šė½ųŠŠŅ»“ĪŹµŃ锣ŹµŃé·½°øĪŖ ”£
ŹµŃé¢ņ£ŗ·“Ó¦²śĪļµÄ¶ØĮæĢ½¾æ
£Ø6£©ŌŚĶÓėÅØĮņĖį·“Ó¦µÄ¹ż³ĢÖŠ£¬·¢ĻÖÓŠŗŚÉ«ĪļÖŹ³öĻÖ£¬¾²éŌÄĪÄĻ×»ńµĆĻĀĮŠ×ŹĮĻ”£
׏ĮĻ1£ŗ
| ĮņĖį/mol”¤L£1 | ŗŚÉ«ĪļÖŹ³öĻÖµÄĪĀ¶Č/”ę | ŗŚÉ«ĪļÖŹĻūŹ§µÄĪĀ¶Č/”ę |
| 15 | Ō¼150 | Ō¼236 |
| 16 | Ō¼140 | Ō¼250 |
| 18 | Ō¼120 | ²»ĻūŹ§ |
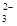 £«I2===S4O
£«I2===S4O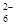 £«2I££©
£«2I££©²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com