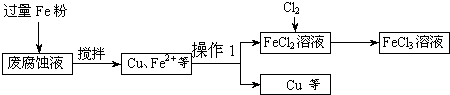
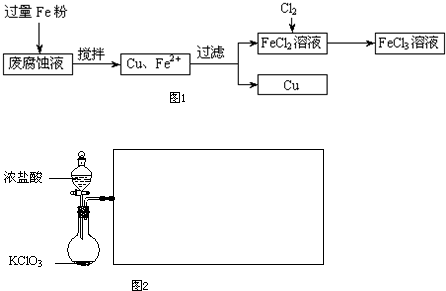
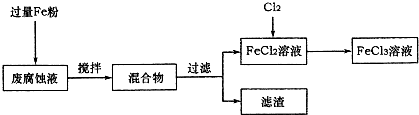
ӡˢ��·�ķϸ�ʴҺ���д���CuCl
2��FeCl
2��FeCl
3�������ŷŽ����»�����Ⱦ����Դ���˷ѣ��ɴӸ÷�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl
3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�ã�
��1�����ij�ϸ�ʴҺ�к�CuCl
2 1.5mol?L
-1��FeCl
23.0 mol?L
-1��FeCl
3 1.0mol?L
-1��HCl3.0mol?L
-1��ȡ�ϸ�ʴҺ200mL������������ʵ���ҽ���ʵ�飺
�ش��������⣺
�ٷϸ�ʴҺ�м���������ۺ�����Ӧ�����ӷ���ʽΪ
2Fe3++Fe=3Fe2+��Cu2++Fe=Fe2++Cu��2H++Fe=Fe2++H2��
2Fe3++Fe=3Fe2+��Cu2++Fe=Fe2++Cu��2H++Fe=Fe2++H2��
��
�ڼ���ϸ�ʴҺ�к���Fe
3+��ʵ�������
ȡ�����ϸ�ʴҺ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+
ȡ�����ϸ�ʴҺ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+
��
�����������У������ˡ��õ��IJ����������ձ�����������
©��
©��
��
���������õ���ͭ�����������Լ���
����
����
��
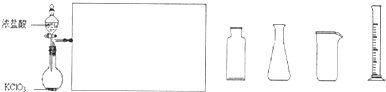
��2��ij��ѧ��ȤС��������ͼװ����ȡ������ͨ�뵽FeCl
2��Һ�л��FeCl
3��Һ��
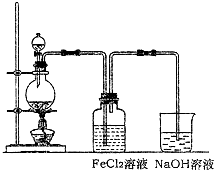
��ʵ�鿪ʼǰ��ijͬѧ��ʵ��װ�ý����������Լ�飬������
�رշ�Һ©�������������ܲ���ˮ�У�����������ƿ�����ܿ�������ð�����ɿ��ֵ����ڳ���һ��ˮ����֤������������
�رշ�Һ©�������������ܲ���ˮ�У�����������ƿ�����ܿ�������ð�����ɿ��ֵ����ڳ���һ��ˮ����֤������������
��
��Ũ������������̷�Ӧ�Ļ�ѧ����ʽΪ
MnO
2+4HCl��Ũ��
��MnCl
2+Cl
2��+2H
2O
MnO
2+4HCl��Ũ��
��MnCl
2+Cl
2��+2H
2O
��
�ձ���NaOH��Һ��������
���ն�����������ֹβ������
���ն�����������ֹβ������
��
�۲ο���1�������ݣ����������̲��������ȡFe�۵�����Ӧ������
39.2
39.2
g����ͨ��Cl
2�����ʵ���Ӧ������
0.75
0.75
mol��





 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�