
��̫������Ч��ת��Ϊ�����ǻ�ʯȼ�Ϻľ�����������Դ����Ĺؼ�����������Խ���Ƭ��ϩΪ���ʽ�̫����ת��Ϊ���ܵķ����������ý���Ƭ��ϩ����̫����ת��Ϊ��ͬ���칹���Ļ��飬�����Ļ����ڴ���������������ת��Ϊ����Ƭ��ϩ���ų����������ɵĽ���Ƭ��ϩѭ��ʹ�ã�
(1)����Ƭ��ϩ�ļ���ʽ��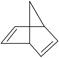 �������ʽΪ________��
�������ʽΪ________��
(2)д������Ƭ��ϩ��H2�����ӳɷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________.
(1)C7H8 (2) ��2H2
��2H2
��������
���������(1)��������Ƭ��ϩ�ļ���ʽ���߸��۵㣬��������������߸�̼ԭ�ӣ�ֻ�����϶˵�̼ԭ������������ԭ�ӣ����������̼ԭ����ֱ�����һ����ԭ�ӣ����Խ���Ƭ��ϩ�ķ���ʽΪC7H8.
(2)����Ƭ��ϩ���ӽṹ�к���̼̼˫��������H2�����ӳɷ�Ӧ
���㣺�����л��ﻯѧʽ�Լ�����ʽ����д
�������������е��Ѷȵ����⣬����ע�ػ��������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������Ĺؼ�����ȷ����ʽ�Ľṹ�ص㣬��ν������������ü��ɣ�����������ѧ����������������֪ʶ��Ǩ��������


 �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л���ѧ��һ����Ҫ���л��ϩ����C��C����һ���Ҽ���һ���м���ɣ����ЦҼ�������ʵĻ����Ǽܣ�������ת�����м��ȦҼ�����������ת��
��ϩ���뵥ϩ����Ȳ�������ʿɷ���1��4���ɻ��ӳɷ�Ӧ��������Ԫ��״������磺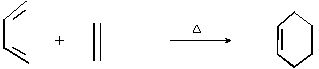
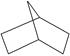
��̫������Ч��ת��Ϊ������ʽ�������ǽ����ʯȼ�Ϻľ���������Դ����Ĺؼ�����������Խ���Ƭ��ϩ��BCD��Ϊ���ʣ���̫����ת��Ϊ������ʽ�������ķ����ǣ�������BCD����̫���⣬BCDת��Ϊ��ͬ���칹���Ļ��飨QC����Ȼ��QC�ڴ���������������ת��ΪBCD�����ų����������ɵ�BCD��ѭ��ʹ�á�
BCD����һ���ӻ����ϩ��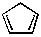 ����һ������Ȳ�����۷�Ӧ�Ƶã�BCD���չ��ܷ����⻯ѧ��Ӧ����QC�Ĺ�����δ�����Ҽ��Ķ��ѡ�
����һ������Ȳ�����۷�Ӧ�Ƶã�BCD���չ��ܷ����⻯ѧ��Ӧ����QC�Ĺ�����δ�����Ҽ��Ķ��ѡ�
����������Ϣ����ش��������⣺
��1�������ϩ�ķ���ʽ��__________��д�������ϩ��һ��ͬ���칹�壨��״�л���Ľṹ��ʽ��__________��
��2���Ƚ�BCD��QC��������Ըߵͣ�BCD_____QC�����������������������
��3��д��BCD��QC�Ľṹ��ʽ��BCD___________�� QC__________��
��4��QC��һ��ȡ������__________���칹�塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com