
����A��B��C��D���ֶ�����Ԫ�أ���֪A��Bͬ���壬B��C��Dͬ���ڣ�A��B��ԭ������֮�͵���C��D��ԭ������֮�ͣ�C�ĵ����ֱܷ��B��D������������ˮ���ﷴӦ����ش�
��1��B��C��Ԫ�ط��ŷֱ�Ϊ_____________________
��2����������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ__________________(��Ԫ�ط��ű�ʾ)
��3��D������A������ȼ�յIJ����������̼��Ӧ�Ļ�ѧ����ʽΪ_____________________
��4��B�����ܸ�D�����������ˮ�����Ũ��Һ����������ԭ��Ӧ�����ɵ������ε�ˮ��Һ���ʼ��ԣ���������ԭ��Ӧ�����ӷ���ʽΪ_____________________________
��1�� S��Al ��2�� Na��Al��S��O ��3�� 2Na2O2 + 2CO2 = 2Na2CO3 + O2 ��4�� 3S + 6OH�C = 2S2�C + SO32�C + 3H2O
�����������������1��C�ĵ����ֱܷ��B��D������������ˮ���ﷴӦ����֪CΪAl��D������A������ȼ�յIJ����������̼��Ӧ��D��Na��A��O������A��Bͬ���壬����BΪS��O��S��ԭ������֮��Ϊ24��Al��Na��ԭ������֮��ҲΪ24��������ȣ��ʿ���ȷ��A��B��C��D����Ԫ�طֱ�Ϊ�����������ơ���B��C��Ԫ�ط��ŷֱ�Ϊ��S��Al����2��Na��Al��Sλ��ͬ���ڣ�����ԭ������������뾶��С������Na��Al��S��O��Sλ��ͬ���壬ԭ������Խ�뾶Խ������S��O���ʴ�Ϊ��Na��Al��S��O����3��D������A������ȼ�յIJ���ΪNa2O2����CO2��Ӧ����Na2CO3��O2����Ӧ�Ļ�ѧ����ʽΪ��2Na2O2 + 2CO2 = 2Na2CO3 + O2����4����������ŨNaOH��Һ��Ӧ�����������ε�ˮ��Һ�Լ��ԣ�˵�����ɵ���������ǿ���Σ���Ԫ���γɵ�������������������ᣬ���ǵ���ӦΪ������ԭ��Ӧ�����ȷ������Na2S��Na2SO3����Ӧ�Ļ�ѧ����ʽΪ��3S + 6NaOH = 2Na2S + Na2SO3 + 3H2O����Ϊ���ӷ���ʽ��3S + 6OH�C = 2S2�C + SO32�C + 3H2O��
���㣺����Ԫ�����ڱ���λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�������Ϣ�ͻ�ѧ����ʽ����д��


 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��Ϊ������Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�B������������ˮ����������⻯�ﷴӦ�γ����ӻ�����ף�A��D������ԭ�Ӹ�����4��1�γɻ������ң����ҷ����к���18�����ӣ�E��Bͬ���壬C����������F�����������һ�����Ӳ㣬�ҿ��γ����Ӹ�����Ϊ2��1�����ӻ��������
(1)D��ԭ�ӽṹʾ��ͼΪ ________ �����ĵ���ʽΪ___________ ��E�����ڱ��е�λ��Ϊ ��
(2)����˵����ȷ���� ��
�ٻ������ҷ�����ֻ���м��Թ��ۼ�
��C��D��E��Fԭ�Ӱ뾶�ɴ�С��˳��ΪC>D>E>F
��B��E�γɵ��⻯���У�B���⻯����ȶ�
�ܻ�����ͻ���������������Ӽ����ۼ�
(3)��Fȼ�յIJ���ͨ��BaCl2��HNO3�Ļ����Һ�У����ɰ�ɫ�������ų���ɫ���壬����һ�����ӷ���ʽ��ʾ�÷�Ӧ __________________ ��
(4)д��һ��������Ԫ�ع��ɵ�10��������18��������Ӧ�����ӷ���ʽ ____________________________ ��
(5)����Һ�� (����ԡ��������ԡ������ԡ�)��ԭ���� (�����ӷ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��X��Y��ZԪ�ص�ԭ������������������Ϣ������⣺
| Ԫ��A | ���ܼ��ϵĵ�������� |
| Ԫ��C | ij�ֺ���ԭ�ӵ�������Ϊ18��������Ϊ10 |
| Ԫ��X | ���������õİ뵼����� |
| Ԫ��Y | �䵥��Ϊ����ɫ���壬���������������ˮ���¶ȼ� |
| Ԫ��Z | 3d�ܼ�����4��δ�ɶԵ��� |
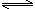 H+(aq)+CH3COO��(aq) ��H��+akJ/mol
H+(aq)+CH3COO��(aq) ��H��+akJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E�Ƕ����ڵ���������Ԫ�ء�AԪ�ص�ij��ԭ�ӣ������û�����ӣ�BԪ�صĻ�̬ԭ��2p�����ֻ��һ�ԳɶԵ��ӣ�C��B����ͬһ���ڣ���ԭ�Ӱ뾶С��B��D��C��ͬ����Ԫ�أ�EԪ�ص�һ�������Ӻ�CԪ�ص������Ӿ�����ͬ�ĵ��Ӳ�ṹ������������Ϣ�ش��������⣺
��1��BԪ�ص�ԭ�ӽṹʾ��ͼ���������� ������CԪ��ԭ�ӵĵ����Ų�ͼ����������������������DԪ��ԭ�ӵļ۵����Ų�ʽ������������������
��2��AԪ�طֱ���C��D��Ԫ���γɵĻ�������ȶ�����ǿ������˳���ǣ��û�ѧʽ��ʾ����������������3����B��D��E����Ԫ����ɵ�ij�ֻ������ˮ��Һ��ʹpH��ֽ�ȱ�������ɫ��д���û�����Ļ�ѧʽ��������������һ�����ӷ���ʽ�����û�����ˮ��ҺʹpH��ֽ�ȱ�������ɫ��ԭ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һЩԪ�ص���Ϣ��������һ��Ԫ�ز��ڶ����ڡ�
| Ԫ��A | Ԫ��B | Ԫ��C | Ԫ��X | Ԫ��Y |
| ������һ�ֳ�����������Ԫ��X�γɺ�ɫ�ͺ���ɫ���ֳ��������� | ��̬ԭ��M��p�������5������ | �������н�������ǿ����X��Ӧ���������ֳ��������� | �������������ڲ��������3�������γ�˫ԭ�������� | ����Ϊ˫ԭ�ӷ��ӣ��ṹ��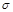 ���� ���� ��Ŀ��Ϊ1:2 ��Ŀ��Ϊ1:2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��D ͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԡ��ش��������⣺
��1������Ԫ���У�ԭ�Ӱ뾶������ ���ǽ�������ǿ���� ����Ԫ�ط��ţ���
��2����A��B��D��E���γɵĹ����ͻ������У����ȶ��������� ���û�ѧʽ��ʾ����
��3��A��E�γɵĻ�������A��B�γɵĻ����ﷴӦ������Ļ�ѧʽΪ �����д��ڵĻ�ѧ������Ϊ ��
��4��D����������ˮ����Ļ�ѧʽΪ ��
��5������D�ڳ���ĵ���E��ȼ�գ���Ӧ�Ļ�ѧ����ʽΪ ��D�ڲ������E��ȼ�գ����ɵ���Ҫ����Ļ�ѧʽΪ ��
��6������E��ˮ��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
18O��ԭ�ӽṹʾ��ͼ �������������� ����������18O2��16O2���е�ԭ����֮���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ�����У�T��������������������������ȣ���ش��������⣺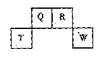
��1��T��ԭ�ӽṹʾ��ͼΪ
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������Q ______ W(�ǿ�ڡ������ڡ�)��
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ ��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ�� ��
��5��R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0��5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ��
��6����298K�£�Q��T�ĵ��ʸ�1mol��ȫȼ�գ��ֱ�ų�����aKJ��bKJ����֪һ�������£�T�ĵ����ܽ�Q������������������û������������û���Ӧ����3molQ�ĵ��ʣ���÷�Ӧ��298K�µ�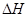 = ������������ ( ע���������浥�ʾ�Ϊ���ȶ�����)��
= ������������ ( ע���������浥�ʾ�Ϊ���ȶ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��A��B��C��D��E���ֶ���������Ԫ���У�A��B��C����Ԫ�ص�ԭ��������������A��C������B��ԭ�Ӹ�����Ϊ1:1��2:1�γɵĻ����D��ԭ��������������࣬E�ĺ˵�������D��E���γ���̬����ED4��
��1������D��ԭ�ӽṹʾ��ͼ________������Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳����________(��Ԫ�ط���)��
��2��A��B�γɵĻ������У����Ǽ��Լ��Ļ�����ĽṹʽΪ ��
��3��C��B��ԭ�Ӹ�����Ϊ1:1�γɻ�����ĵ���ʽ�� ��
��4��D��E���γ��⻯����ȶ�����ǿ������˳���� �� (�����Ļ�ѧʽ)��
��5���ɶ�����Ԫ����ɵ�ijЩ������SO2��O3��NO2-�ɻ���Ϊ�ȵ����壬����B��Dͬ����Ԫ����ɵ����У�����N3-��CS2����Ϊ�ȵ���������� (�����Ҫ��������������)��
��6��д����ҵ����E���ʵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com