
| A������ʱ��ij��Һ����ˮ���������c��H+����c��OH-���ij˻�Ϊ1��10-24������Һ��һ�����Դ�������K+��Na+��AlO2-��SO42- | ||
B������ʱ��0.1mol/L HA��Һ��pH��1��0.1mol/L BOH��Һ��
| ||
C������SO2ͨ�뵽Ba��NO3��2��Һ�У���ȷ�����ӷ�Ӧ����ʽΪ��3SO2+2NO3-+Ba2++2H2O�TBaSO4��+2NO��+4H++2S
| ||
| D�������£�ϡ��0.1mol/L�İ�ˮ����Һ��c��OH-����c��NH4+����c��H+�����½� |
| c(OH-) |
| c(H+) |
| c(OH-) |
| c(H+) |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
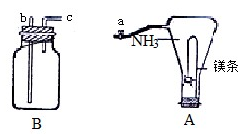 ȫ��þ���к�ǿ�Ļ�ԭ�ԣ���ȼ��þ�����ڰ����о���ȼ�գ��������·�Ӧ��
ȫ��þ���к�ǿ�Ļ�ԭ�ԣ���ȼ��þ�����ڰ����о���ȼ�գ��������·�Ӧ��| ��ȼ |
| ��ȼ |
| nMg(NH2)2 |
| nMgNH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ԫ�ص�ͬ�����Ԫ�ص�ԭ�ӣ�������������ͬ������ѧ���ʲ���ܴ� |
| B����Ԫ��λ�ڳ����ڣ��Ҹ���������32��Ԫ�� |
| C����Ԫ�ص����������������ӹ��� |
| D����Ԫ��Ϊ53��Ԫ�أ������ԭ����Ϊ127�����Ԫ�ص�һ��ԭ���бض�����74������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
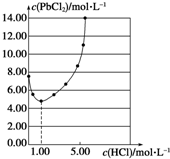 25��ʱ��PbCl2�����ڲ�ͬŨ�������е��ܽ����ͼ�����Ʊ�PbCl2��ʵ���У�ϴ��PbCl2�������ѡ�ã�������
25��ʱ��PbCl2�����ڲ�ͬŨ�������е��ܽ����ͼ�����Ʊ�PbCl2��ʵ���У�ϴ��PbCl2�������ѡ�ã�������| A������ˮ |
| B��1.00 mol?L-1���� |
| C��5.00 mol?L-1���� |
| D��10.00 mol?L-1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ʊ����ᶡ�������������������ᣬ�ٵμӼ���Ũ���ᣬ��ˮԡ���� |
B�� ���������Һ���Ƿ��������̪������ͼ��ʾ����ֽ�ϲ�����ʵ�� ���������Һ���Ƿ��������̪������ͼ��ʾ����ֽ�ϲ�����ʵ�� |
| C������ȩ��������CuSO4��Һ��NaOH��Һ��1mL����������ȩ��Һ����� |
| D���Ƚϱ��ӡ����ᡢ̼������ԣ�����ʹ��Ӧ����������ͨ�뱽������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D | |
| X | �� | ���������� | �� | ���� |
| Y | �� | �� | �� | �� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��2 | B��1��4 |
| C������1��4 | D����1��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com