
[s1]  ��һ���º�ѹ�ܱ������У�A��B����ɽ�������ƽ�⣺2A(g)+2B(g)
C(g)+3D(g)�ֱַ������;������ƽ�⣺I��A��B����ʼ����Ϊ2mol��II��C��D����ʼ���ֱ�Ϊ2mol��6mol������������ȷ���� �� ��
��һ���º�ѹ�ܱ������У�A��B����ɽ�������ƽ�⣺2A(g)+2B(g)
C(g)+3D(g)�ֱַ������;������ƽ�⣺I��A��B����ʼ����Ϊ2mol��II��C��D����ʼ���ֱ�Ϊ2mol��6mol������������ȷ���� �� ��
A���ﵽƽ��ʱ��;��I�������ܶ�Ϊ;��II�ܶȵ�1/2
B��I��II��;�����մﵽƽ��ʱ����ϵ�ڸ������Ũ�Ȳ�ͬ
C���ﵽƽ��ʱ��;��I��;��II��ϵ�ڻ������ƽ����Է���������ͬ
D��I��II��;�����մﵽƽ��ʱ����ϵ�ڻ������İٷ������ͬ
[s1]8��
 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ɽ��ģ��2012��2��27���������ƽ��롰200��������ʱ����������β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��2012?��ɽ��ģ��2012��2��27���������ƽ��롰200��������ʱ����������β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��������ɽ��������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
2012��2��27���������ƽ��롰200��������ʱ����������β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��1��������ȼ������ʱ����Ӧ��N2(g)+O2(g) 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����2L�ܱ������г���4mol N2��6molO2��5min���ƽ��ʱNO���ʵ���Ϊ4mol���÷�Ӧ������v(N 2)Ϊ ������������µ�ƽ�ⳣ����д��������̣���
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����2L�ܱ������г���4mol N2��6molO2��5min���ƽ��ʱNO���ʵ���Ϊ4mol���÷�Ӧ������v(N 2)Ϊ ������������µ�ƽ�ⳣ����д��������̣���
��2�����º��ݣ���˵����Ӧ 2NO(g)  N2(g)+O2(g) �ﵽƽ����� ������ţ���
N2(g)+O2(g) �ﵽƽ����� ������ţ���
A��NO��N2��O2��Ũ��֮��Ϊ2��1��1
B��N2��Ũ�Ȳ��ٷ����仯
C����λʱ��������2 mol NO��ͬʱ����1 mol N2
D�������������ܶȲ��ٷ����仯
��3��H2��CO���Դ���ԭNO�Դﵽ������Ⱦ��Ŀ�ģ�
����֪��N2(g)+ O2(g) = 2NO(g) ��H = +180.5kJ/mol
H2(g)+1/2O2(g) = H2O(l) ��H = ��285.8kJ/mol
��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽΪ ��
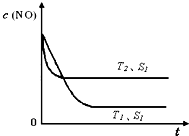
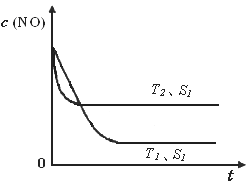
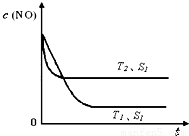
�ڵ�����һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ��2NO(g) + 2CO(g)  2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��ݴ��жϸ÷�Ӧ�ġ�H
0 (�����������������ȷ����)���������ı����S1��S2
������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��ݴ��жϸ÷�Ӧ�ġ�H
0 (�����������������ȷ����)���������ı����S1��S2
������ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�ϲ��а�һ��ѧ���鶼��ѧ��������ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[s1] ![]() ��һ���º�ѹ�ܱ������У�A��B����ɽ�������ƽ�⣺2A(g)+2B(g) C(g)+3D(g)�ֱַ������;������ƽ�⣺I��A��B����ʼ����Ϊ2mol��II��C��D����ʼ���ֱ�Ϊ2mol��6mol������������ȷ���� �� ��
��һ���º�ѹ�ܱ������У�A��B����ɽ�������ƽ�⣺2A(g)+2B(g) C(g)+3D(g)�ֱַ������;������ƽ�⣺I��A��B����ʼ����Ϊ2mol��II��C��D����ʼ���ֱ�Ϊ2mol��6mol������������ȷ���� �� ��
A���ﵽƽ��ʱ��;��I�������ܶ�Ϊ;��II�ܶȵ�1/2
B��I��II��;�����մﵽƽ��ʱ����ϵ�ڸ������Ũ�Ȳ�ͬ
C���ﵽƽ��ʱ��;��I��;��II��ϵ�ڻ������ƽ����Է���������ͬ
D��I��II��;�����մﵽƽ��ʱ����ϵ�ڻ������İٷ������ͬ
[s1]8��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com