
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm
3�����Ƴ�250mL 0.1mol?L
-1��������Һ��
��1����ͼ��ʾ�����У�����������Һ����Ҫ����
������ͼ��Ӧ��������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ�
��������
�������ð�ʹ�õ��Ⱥ�˳��ֱ���_
��
��
��2�����ݼ��㣬�������̻����У���ʵ����ͲӦʹ�õ���
������ƿӦʹ��
�����ں�������д��Ӧ����ţ������֣���ͬ��
A��10mL B��100mL C��250mL D��500mL
��3��������ƿ��ʹ�÷����У����в�����ȷ����
��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ��ˮϴ����������õ�ϡHCl��Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶���
D��������Һʱ����������Һ�壬����Ͳȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ��
��4�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ��
A��ֱ��ת�Ƶ�ϸ���Լ�ƿ�� B�����ý�ͷ�ιܼ�ˮ�����¶���
C��������Һ�������������� D��ֱ������������ƿ��
��5����������ʱ������������ȷ��ֻ��������ijһ��������ж������Ƶ���ҺŨ�������Ҫ���ֵ����0.1mol/L����Σ���a��ƫ�ߣ�b��ƫ�ͣ�c����Ӱ�죬�����к���������Ӧ��ţ�
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ��
��������ʱ���ӣ�������Һ�����ʵ���Ũ��
��



 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�

 �Ʊ���
�Ʊ��� ��2����ԭ�ӵķ������ĺϳ�·��
��2����ԭ�ӵķ������ĺϳ�·��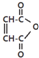 ��һ���л��Լ����л��ϳ�ԭ�ϣ��Ƕ�ϩ���������������1��3-����ϩΪԭ����ȡ��ϩ���ᣨHOOCCH=CHCOOH���������������·�������С�������ָ��ǿ�����������ǻ�����Ϊ�Ȼ�����
��һ���л��Լ����л��ϳ�ԭ�ϣ��Ƕ�ϩ���������������1��3-����ϩΪԭ����ȡ��ϩ���ᣨHOOCCH=CHCOOH���������������·�������С�������ָ��ǿ�����������ǻ�����Ϊ�Ȼ����� X��Y��Z��WΪ�����ڵ�����Ԫ�أ���ԭ��������������XԪ���γɵĵ�������Ȼ���к����������壮Y�ǵ縺������Ԫ�أ�W��ԭ������������������������֮��Ϊ3��8��X��ԭ��������Z��ԭ��������һ�룮Uԭ�ӻ�̬����Χ�����Ų�3d104s1��
X��Y��Z��WΪ�����ڵ�����Ԫ�أ���ԭ��������������XԪ���γɵĵ�������Ȼ���к����������壮Y�ǵ縺������Ԫ�أ�W��ԭ������������������������֮��Ϊ3��8��X��ԭ��������Z��ԭ��������һ�룮Uԭ�ӻ�̬����Χ�����Ų�3d104s1��
 ��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��