
ijС��ȡһ��������Fe SO4����,����ͼ��װ�ý���ʵ�顣
SO4����,����ͼ��װ�ý���ʵ�顣
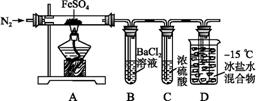
| ʵ����� | ʵ������ | |
| �� | ͨ��һ��ʱ��N2,���� | A�й����Ϊ����ɫ,B���а�ɫ����,D�Թ�������ɫҺ�� |
| �� | �ô��л��ǵ�ľ������װ��D�ĵ��ܿ� | ľ����ȼ |
| �� | ��ַ�Ӧ, | �����ܽ�,��Һ�ʻ�ɫ |
| �� | ����������Һ����D�Թ��� | ��Һ��Ϊdz��ɫ |
��֪:SO2�۵�Ϊ-72 ��,�е�Ϊ-10 ��;SO3�۵�Ϊ16.8 ��,�е�Ϊ44.8 �档
(1)ʵ��۷�Ӧ�����ӷ���ʽ����������������������������������������
(2)�ֽ���̳�����ʹľ����ȼ��������,����A�й�����ɫ�仯�Ʋ�,��һ������������,��������������
(3)ʵ��ܷ�Ӧ�����ӷ���ʽ��������������������������������������������������������
(4)ijͬѧ����B�е�����,��ΪFeSO4 �ֽ�һ����SO3���ɡ�����Ϊ�Ƿ���ȷ,��ԭ����������������(�ñ�Ҫ�����ֺͻ�ѧ����ʽ����)��
�ֽ�һ����SO3���ɡ�����Ϊ�Ƿ���ȷ,��ԭ����������������(�ñ�Ҫ�����ֺͻ�ѧ����ʽ����)��
�淶����:(1)Fe2O3+6H+ 2Fe3++3H2O
2Fe3++3H2O
(2)SO2����Ϊ��Fe2O3����,��FeSO4��ֻ��+6��SԪ����������,�ܱ���ԭ�����һ����SO2����
(3)2Fe3++SO2+2H2O 2Fe2++S+4H+
2Fe2++S+4H+
(4)����ȷ,��Ϊ�ֽ���O2 ��SO2����,��ˮ��Һ�з�����Ӧ:2SO2+O2+2H2O
��SO2����,��ˮ��Һ�з�����Ӧ:2SO2+O2+2H2O 2H2SO4,�����۷ֽⷴӦ�Ƿ���SO3����,�����д�����(��д2SO2+O2+
2H2SO4,�����۷ֽⷴӦ�Ƿ���SO3����,�����д�����(��д2SO2+O2+ 2H2O+2BaCl2
2H2O+2BaCl2 2BaSO4��+4HClҲ��)
2BaSO4��+4HClҲ��)
����:(1)A�к���ɫ����ΪFe2O3,���������,����FeCl3��(2)�������й������ɫ��֪FeԪ�ر�����,��Ӧ������һ��Ԫ�ر���ԭ,��ֻ����+6�۵���Ԫ�ر���ԭ,�õ�����SO2��(3)����֪���ʵ��۷е��֪,D�к���Һ̬��SO2,��Fe3+���䷢��������ԭ��Ӧ��


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������أ�K2FeO4����һ�ָ�Ч���ˮ�����������м�ǿ�������ԡ�
��1����֪��4FeO42-+10H2O  4Fe��OH��3+8OH-+3O2����K2FeO4�ڴ���ˮ�Ĺ���������������� ��
4Fe��OH��3+8OH-+3O2����K2FeO4�ڴ���ˮ�Ĺ���������������� ��
ͬŨ�ȵĸ��������pHΪ4��74��7��00��11��50��ˮ��Һ�����ȶ�����pH= ����Һ��
��2��������������¼��ֳ����Ʊ�������
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ�������κ�KNO2�Ȳ��� |
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��NaClO��Ӧ�����Ϻ�ɫ����������Һ |
| ��ⷨ | �Ʊ��м����Na2FeO4������KOH��Һ��Ӧ |
�ٸɷ��Ʊ�K2FeO4�ķ�Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ____ ��
��ʪ���Ʊ��У���Fe��NO3��3����������ڼ��Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4���˷�Ӧ�����ӷ���ʽ�� ��
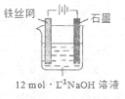
���Ʊ��м����Na2FeO4���ɲ��õ�װ����ͼ��ʾ���������ĵ缫��Ӧ
ʽΪ ��
��3�����ǵ�˫ģ�綯����ʹ�ø�����ع��磬���ܷ�ӦΪ��
3Zn+2K2FeO4+8H2O  3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH
�ŵ�ʱ��������Ϊ ��������ӦΪ�� ��
��4�� 25��ʱ��CaFeO4��Ksp=4��54��l0-9����Ҫʹ1000 L������2��0��l0��4 mol·L-l K2FeO4�ķ�ˮ����CaFeO4�������������������ټ���Ca��OH��2�����ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ������������������B��D���ڳ���������J��һ�ֺ�ɫ���壬I��Ũ��Һ���л�ԭ�ԣ�A��I��Ϊ��ѧ��ѧ�������ʣ�����֮���ת����ϵ����ͼ��ʾ��������������ͷ�Ӧ��������ȥ��
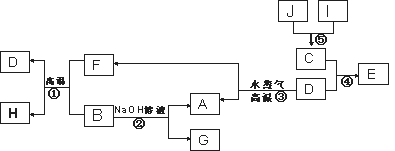
��ش��������⣺
(1) д���������D��Ԫ�������ڱ��е�λ�ã�___________________��
(2) ��д����Ӧ�ٵĻ�ѧ����ʽ��______________________________��
����E�ı�����Һ�����Ƶý��壬�û�ѧ����ʽ��ʾ�ù��̵�ԭ����_______________��
��д����Ӧ�ڵ����ӷ���ʽ_________________________________��
(3) ��֪G��ˮ��Һ�Լ��ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��_________��
(4) J��H2O2�ֽⷴӦ������������������J�����ữ��H2O2��Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��___________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
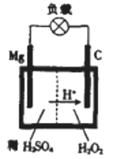
1808�꣬Ӣ����ѧ���üػ�ԭ����þ�������Ƶ�������þ��þ�Ǻ��չ�ҵ����Ҫ���ϣ�þ��Ϊһ��ǿ���������������ѡ�����˵������С�
��1��þ��Ԫ�����ڱ��е�λ��Ϊ_________________________.
(2)д����þ����ʯ����Ҫ�ɷ�ΪTi O2���ڼ�����������ȡ�ѵĻ�ѧ����ʽ��_____________________________________.
(3)þ�ڼ��ȵ������»�����NaOH���巴Ӧ������MgO�͵���X������Y����֪X��ˮ��Ӧ�����ɵ���Y����þ��NaOH��Ӧ�Ļ�ѧ����ʽΪ____________________.
(4)þ—���������صĹ���ԭ����ͼ��ʾ���õ�طŵ�ʱ�ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z�����ֳ���Ԫ�صĵ��ʣ����������ֳ����Ļ������Щ���ʺͻ�����֮�������ͼ��ʾ�Ĺ�ϵ������˵����ȷ���� �� �� 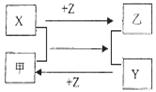
A��X��Y��Z���Ƿǽ�������
B��X��Y��Z��������һ���ǽ�������
C�����X��Y��Ϊ�ǽ������ʣ���Z��Ϊ��������
D�����X��Y��Ϊ�������ʣ���Z��Ϊ�ǽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������(��Ҫ�ɷ���Al2O3)�������Ĺ�������ʾ��ͼ����:

(1)������ɵ���������Һ������(��ϲ㡱���²㡱),���ʱ�������ĵĵ缫������(���������������)��
(2)д��ͨ�����������̼�ữʱ��Ӧ�����ӷ���ʽ:������������������������������������������
(3)����� ����ʱ,��������ʯ(Na3AlF6),����
����ʱ,��������ʯ(Na3AlF6),���� ��������������������������,��ҵ�Ͽ����÷��������塢���������ʹ����ڸ��������·�����Ӧ����ȡ����ʯ,д���÷�Ӧ�Ļ�ѧ����ʽ:����������������������
��������������������������,��ҵ�Ͽ����÷��������塢���������ʹ����ڸ��������·�����Ӧ����ȡ����ʯ,д���÷�Ӧ�Ļ�ѧ����ʽ:����������������������
(4)����������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ��ص���������������(�ѧʽ),�����ĵ缫��ӦΪ�������������������� ������
������
(5)�Խ�����Ʒ���п���ʴ����,���ӳ���ʹ��������
�ٿ���һ���������е��(����ͼ),��ʱ��������γ��������������Ĥ,��缫��ӦΪ����������;

�ڸֲĶ�����,�ܷ�ֹ�ֲĸ�ʴ,��ԭ��������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC10H20O2���л��������������¿�ˮ��Ϊ��A����B��A��������������ת��ΪB���������������칹����������Ҫ��Ĵ�������������ϣ����γɵ������У��� ��
A��32 B��16 C��8 D��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC2H4O2�Ľṹ������ ��
�� ���֣�Ϊ����ṹ����������������������______________��________________��
���֣�Ϊ����ṹ����������������������______________��________________��
(1)��Ϊ ������������Ӧ����____�������գ��˴Ź���������Ӧ����__________���塣
������������Ӧ����____�������գ��˴Ź���������Ӧ����__________���塣
(2)��Ϊ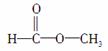 ��������������______�������գ��˴Ź���������Ӧ��______���塣
��������������______�������գ��˴Ź���������Ӧ��______���塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������(NaClO2)��һ����Ҫ������������֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2·3H2O����ClO2�ķе�Ϊ283K����ClO2�ֽⱬը����HClO2��25��ʱ�ĵ���̶�������ĵڶ�������̶��൱������Ϊǿ�ᡣ��ͼ�ǹ������ⷨ�����������ƵĹ�������ͼ��

(1)C1O2����������������Ӧ�����ӷ���ʽΪ ���������й�����������ÿ����� (ѡ�����)��
A����SO2������SO3��ǿ���� B��ϡ��C1O2�Է�ֹ��ը
C����NaClO3������C1O2
(2)�ڸ�ʵ����������Ũ������ʾNaOH��Һ����ɣ���ʵ��ʱ��Ҫ450ml
l60g��L��NaOH��Һ�����ھ�ȷ����ʱ����Ҫ��ȡNaOH�������� g��
��ʹ�õ�������������ƽ����Ͳ���ձ����������⣬��������
(3) �������ڵ��¶Ȳ��ܳ���20�棬����ҪĿ���� _���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ ��
(4)���������У��ɴ���H2O2���Լ��� (�����)��
A��Na2O2 B��Na2S C��FeCl2 D��KMnO4
(5)����Һ�еõ�NaClO2·3H2O�����ʵ����������� ����������ƣ�
A������ B������Ũ�� C������ D������ E����ȴ�ᾧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com