
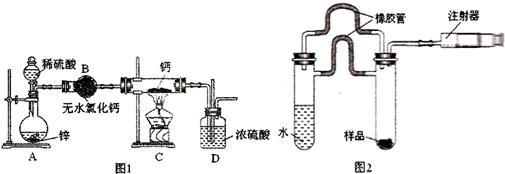
���� ��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը��
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y�����ݷ���ʽCaH2+2H2O�TCa��OH��2+2H2����Ca+2H2O�TCa��OH��2+H2�����з��̼���x��y��ֵ���ٸ�����������������㣻
��6����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
��� �⣺��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���װ��B�������ǣ���ȥ�����е�ˮ������װ��D�������ǣ���ֹ�����е�ˮ��������Cװ�ã�
�ʴ�Ϊ����ȥ�����е�ˮ��������ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը������ȷ�IJ���˳��Ϊ���ڢ٢ܢۣ�
�ʴ�Ϊ���ڢ٢ܢۣ�
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
�ʴ�Ϊ����ˮ����ͭ��
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
�ʴ�Ϊ��Na2CO3��ϴ�ӣ���ɣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y����
CaH2+2H2O�TCa��OH��2+2H2��
42 4
X Y
����42��4=x��y��������y=$\frac{2x}{21}$
Ca+2H2O�TCa��OH��2+H2��
40 2
46mg-x 4.2mg-y
����40��2=��46mg-x������4.2mg-y������y=$\frac{2x}{21}$���룬���x=42mg��������Ʒ���⻯�ƵĴ���Ϊ��$\frac{42mg}{46mg}$��100%=91.30%��
�ʴ�Ϊ��91.30%��
��6����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
�ʴ�Ϊ����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
���� �������⻯���Ʊ�Ϊ���壬����ʵ�����������������Ժ����е���Ϣ�����á���ʵ��װ�õ�������������ʷ����ᴿ����ѧ���㡢ʵ�鷽����Ƶȣ�������ػ���ʵ������������飬�Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ƽ� | B�� | ��ù�� | C�� | ��������� | D�� | ţ�ƽⶾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2•6C2H5OH��
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2•6C2H5OH���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
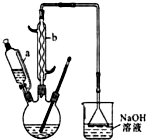 ������
������ 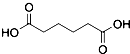 ��һ�ֹ�ҵ�Ͼ�����Ҫ������л���Ԫ�ᣬ�ڻ����������л��ϳɹ�ҵ��ҽҩ��������ȷ��涼����Ҫ���ã��ܹ��������η�Ӧ��������Ӧ�ȣ��������Ԫ�����۳ɸ߷��Ӿۺ���ȣ���������������ж�Ԫ�����еĵڶ�λ��ʵ���Һϳɼ�����ķ�Ӧԭ����ʵ��װ��ʾ��ͼ��
��һ�ֹ�ҵ�Ͼ�����Ҫ������л���Ԫ�ᣬ�ڻ����������л��ϳɹ�ҵ��ҽҩ��������ȷ��涼����Ҫ���ã��ܹ��������η�Ӧ��������Ӧ�ȣ��������Ԫ�����۳ɸ߷��Ӿۺ���ȣ���������������ж�Ԫ�����еĵڶ�λ��ʵ���Һϳɼ�����ķ�Ӧԭ����ʵ��װ��ʾ��ͼ��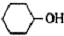 +8HNO3��3
+8HNO3��3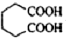 +8NO��+7H2O
+8NO��+7H2O| ���� | �ܶȣ�20�棩 | �۵� | �е� | �ܽ��� | ��Է������� |
| ������ | 0.962g/cm3 | 25.9�� | 160.8�� | 20��ʱˮ���ܽ��3.6g���ɻ������Ҵ����� | 100 |
| ������ | 1.36g/cm3 | 152�� | 337.5�� | ��ˮ�е��ܽ�ȣ�15��ʱ1.44g��25��ʱ2.3g���������Ҵ��������ڱ��� | 146 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
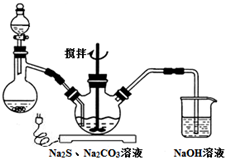 NaCNΪ�綾���ij��ȤС������ϵ�֪��ʵ�������NaCN��Һ��ʹ��Na2S2O3��Һ���нⶾ���٣����ǿ�չ����������ʵ�飬�����Ҫ��ش����⣺
NaCNΪ�綾���ij��ȤС������ϵ�֪��ʵ�������NaCN��Һ��ʹ��Na2S2O3��Һ���нⶾ���٣����ǿ�չ����������ʵ�飬�����Ҫ��ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������������Һ��Al+2OH-�T2AlO2-+H2�� | |
| B�� | AlCl3��Һ�м��������İ�ˮ��Al3++4OH-�TAlO2-+2H2O | |
| C�� | ����������ˮ��Ӧ��Na2O2+2H2O�T2Na++2OH-+O2�� | |
| D�� | FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2�T2Fe3++2Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com